Article Text
Abstract
Objectives This study aimed to compare mortality rates and length of hospital stay between patients with critical COVID-19 transferred to another hospital due to capacity constraints and those who remained at their initial admission hospital.
Design Single-centre cohort study.
Setting and participants 665 patients were treated for SARS-CoV-2 at two intensive care units (ICUs) in Stockholm, Sweden, from 1 March 2020 to 30 June 2021. Data on interhospital transfers (IHTs) were retrieved from medical records and patient data management systems according to predefined protocols.
Main outcome measures The outcomes were 30-day and 90-day mortality, days alive and out of ICU. HR with 95% CI were calculated using Cox proportional hazard models with adjustments for age, sex, body mass index, severity of illness, comorbidity, invasive ventilation, treatment limitations and pandemic waves.
Results Of 665 patients, 133 (20%) were transferred to another hospital. The mortality rate of transferred patients compared with non-transferred patients at 30 days was 19% vs 26% (p=0.13) and at 90 days 26% vs 30% (p=0.43). In the adjusted Cox regression analysis, IHT was associated with a lower mortality risk at 30 days (HR 0.47, 95% CI 0.30 to 0.76) and 90 days (HR 0.52, 95% CI 0.34 to 0.79). However, the number of days alive and out of ICU was significantly lower for the IHT group at 30 days.
Conclusion In our study, IHT due to capacity constraints among critically ill COVID-19 patients was not associated with a higher mortality risk. The suitability for transfer was likely associated with lower mortality, although residual confounding cannot be ruled out. The requirement for invasive ventilation among transferred patients might account for the extended length of ICU stay, rather than the transfer itself. However, the difficulty in studying this issue lies in the fact that while patients are likely exposed to risks during transfer, they are simultaneously the patients stable enough to be transported.
- COVID-19
- Adult intensive & critical care
- Safety
Data availability statement
Data are available on reasonable request. The data that support the findings of this study are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The strengths are the comprehensive data collection conducted by dedicated researchers during the three pandemic waves and validated by independent researchers.
The low proportion of missing data, and the adjustment for known confounders in the analyses.
The main weakness is the single-centre non-randomised design.
There may be a risk of selection bias in the study, since it is likely that respiratory-stable and haemodynamic-stable patients with recovery potential were selected for transport.
Information about the severity of illness immediately before transport was not readily available in our cohort.
Background
Transports of critically ill patients between intensive care units (ICUs) across hospitals are relatively common. The primary reasons include the need to transfer to a hospital with specialised competence, as well as capacity constraints at the transferring hospital.1–3 Regardless of the reason for the transport, studies have shown that patient transfers entail direct risks related to the transport itself and can be linked to delayed diagnostics or treatments.1 4–8 In the years before the COVID-19 pandemic, around 600 ICU patients (1.8%) were transported between hospitals in Sweden each year due to resource shortages at the sending ICU. However, during the pandemic, the influx of patients requiring ICU treatment significantly increased interhospital transfers (IHTs) to 3.5%.9
Studies investigating the risk of IHT during the COVID-19 pandemic have shown conflicting results. Some studies indicate that IHT is not associated with an increased mortality risk2 10–13 or may even be associated with a lower risk of death,14 whereas others have shown that IHT is associated with a longer duration of mechanical ventilation,10 ICU stay11 12 and length of hospital stay.2 11 None of these studies were randomised controlled trials, and many were limited by a small sample size.2 10 13 Despite the large increase in ICU beds in Sweden during the pandemic, a substantial proportion of critically ill patients were transferred between hospitals. To the best of our knowledge, no study has so far studied the influence of IHTs on patient outcomes during the COVID-19 pandemic in Sweden. Therefore, we aimed to compare mortality rates and length of hospital stay between patients transferred to another hospital due to capacity constraints and those who remained at their initial admission hospital.
Methods
Study design
This study included all adult patients (age ≥18 years) with confirmed SARS-CoV-2, as verified by PCR test, requiring intensive care at Södersjukhuset, Sweden, between 1 March 2020 and 30 June 2021. The hospital is a secondary referral centre, serving a diverse range of medical and surgical patients in Stockholm, a city with 2.5 million inhabitants. The hospital has a total capacity of 600 beds and houses a large emergency department with a continuous inflow of acutely ill patients. Healthcare in Sweden is predominantly funded by taxes, ensuring access to medical services for all citizens. Normally, there are 18 beds dedicated to intensive care. However, during the COVID-19 pandemic, the ICUs expanded to accommodate a total of 60 ICU beds. The reporting of the study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.15
Cohort
All adult patients with a SARS-CoV-2 diagnosis and initial admission to the study hospital’s ICU were eligible for inclusion in the study. Patients first admitted to other ICUs and subsequently transferred to the study hospital were excluded. Additionally, patients transferred due to reasons other than capacity constraints, such as repatriation (return to a local ICU near the patient’s home) and clinical transfers (requiring specialised care not available at the admitting hospital) were excluded from the study.
Patients were identified using the patient data management system. Clinical data were automatically and manually extracted from medical records and the patient data management system using a predefined data collection protocol to ensure consistency and uniformity of the data collection. Cross-validation of data from randomly selected patients was performed separately by two independent researchers.
Exposure
The exposure was IHT, specifically defined as the transfer of patients from the ICU at the index hospital to another hospital’s ICU due to capacity constraints. These constraints typically involve a shortage of staffed ICU beds at the referring hospital, necessitating the transfer of patients to ensure they receive the critical care they need.
The IHTs were conducted in accordance with the transport recommendations provided by the Swedish Society for Anaesthesia and Intensive Care. These guidelines ensure that transfers are carried out safely and efficiently, minimising risks to the patient during transport. The guidelines cover various aspects, such as patient stability before the transfer, necessary medical equipment during the transfer and the qualifications of the medical personnel accompanying the patient. The transfers were crucial for managing ICU capacity and ensuring that patients continue to receive appropriate care without delay.16 The selection of patients for transfer was commonly carried out in consultation with the admitting ICU. The decision was based on the patient’s physiological status to ensure safe transport and the receiving hospital’s capacity to provide the required care. This process did not adhere to a standardised protocol or guideline; rather, it relied on the clinical judgement of senior physicians, who made joint decisions about which patient was most suitable for transfer, based on various clinical factors and the urgency of the situation. The decision-making process involved assessing the patient’s condition, stability, and the potential risks and benefits of transfer, aiming to ensure that the patient most suitable for ICU care at another facility was selected for transfer, taking into consideration the overall capacity and resources of both the referring and receiving hospitals. The lack of a standardised protocol meant that decisions were tailored to individual cases relying on clinical expertise and teamwork in managing ICU capacity and patient care.
The transport was prepared according to local routines, and handover was reported by telephone. ICU patients in Stockholm are commonly transported between hospitals using a mobile intensive care unit (MICU). An MICU is staffed with one ambulance nurse, one paramedic and one physician from the departing ICU. The MICU transporter is more spacious than a standard ambulance and is equipped with continuous electrocardiography monitoring, invasive haemodynamic monitoring and advanced ventilatory support. Information about the date, time and reasons for transfer was obtained from the patient’s medical records and the patient data management system.
Outcomes
The outcomes were mortality at days 30 and 90 from ICU admission and days alive and out of ICU. The length of ICU stay was measured from the date of ICU admission to the date of discharge from the ICU at Södersjukhuset or the admitting hospital.
Statistical analysis
Continuous variables are presented as medians with IQRs, and categorical variables are expressed as numbers and proportions (percentages). The distribution of continuous variables was tested with the Shapiro-Wilk test. Differences between groups were analysed using the Mann-Whitney U test and the χ2 test for continuous and categorical variables, respectively.
In the survival analyses, patients were followed up from the date of ICU admission until the date of death or until 30 and 90 days had passed since admission. Kaplan-Meier curves were used to estimate the cumulative risk of death, and the log-rank test was employed to compare patients with exposure to IHT versus those without IHT. Cox proportional hazards regression was used to estimate HR with corresponding 95% CI for death within 30 and 90 days from ICU admission. Multivariate models were adjusted for age (continuous), sex (male/female), body mass index (BMI) (<30/≥30 kg/m2), Simplified Acute Physiology Score III (SAPS III) (>50/50 to 59/≥60), Charlson Comorbidity Index (categorised as 0/1/2),17 invasive mechanical ventilation (yes/no), treatment limitations (yes/no), COVID-19 waves (first wave, March to September 2020 versus second and third waves, October 2020 to January 2021 and February to June 2021)18 and IHT (yes/no). Both crude and multivariable models were investigated with no IHT as the reference group. Scaled Schoenfeld residuals were regressed against survival time to assess the proportional hazard assumptions, and Martingale residual plots, together with the Grambsch-Therneau test, were used to assess evidence of non-linearity. Variance inflation factors were used when investigating multicollinearity. All variables were complete except for SAPS and BMI, which were missing for 105 and 6 patients, respectively. This was handled by a separate category for patients with missing values.
Since the proportional hazards assumption in the Cox regression was not met, indicating that the effect of the variables could vary over time, we performed sensitivity analyses on subgroups of patients based on length of ICU stay (more than 6, 8, 10 and 12 days), COVID-19 waves, mechanical ventilation and treatment limitations. Comparisons of days alive and free from ICU were conducted using the Mann-Whitney U test and presented as medians with IQRs.
A two-sided p value less than 0.05 was considered statistically significant. The analysis followed a predefined protocol and was conducted using the IBM SPSS Statistics V.29 statistical software and R V.4.3.3 (R Core, 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). All analyses were discussed and confirmed with an experienced biostatistician.
Patient and public involvement
Patients and/or the public were not involved in the design or conduct or reporting or dissemination plans of this research.
Results
Patient cohort
Of 2622 critically ill patients admitted to the two ICUs during the study period, 674 had a confirmed SARS-CoV-2 diagnosis. Nine patients were transported for medical reasons such as the need of extracorporeal membrane oxygenation (ECMO) and five patients had been transferred to the study hospital from other hospitals/regions. In total, 665 SARS-CoV-2 patients were included in the study, of which 133 (20%) underwent interhospital transports due to capacity constraints (figure 1). All patients (n=133) were transported to a hospital within a 30 km radius or maximum of 30 min.
Flowchart of study inclusion. ICU, intensive care unit.
The median age of included patients was 64 years, and most were men (72%). Patients who were transferred were more often on mechanical ventilation (98%) compared with the non-transferred patients (40%), and fewer had limitations in terms of life-sustaining treatment (4% vs 15%). Otherwise, the groups were balanced regarding patient and clinical characteristics (table 1). The median day of IHT from admission was 6 (IQR: 3–11).
Characteristics of patients admitted to an ICU for SARS-CoV-2
Interhospital capacity transfer and mortality
Analyses and distribution of IHT and mortality in patients with critical COVID-19 are shown in table 2 and online supplemental file 1. Mortality rates did not differ between groups at either 30 days (19% vs 26%, p=0.13) or 90 days (26% vs 30%, p=0.43). This was consistent across the log-rank test for survival for 30 days (figure 2, p=0.06) and 90 days (figure 3, p=0.2), as well as the HR of the univariate Cox regression of 0.66 (95% CI 0.43 to 1.02) for 30-day mortality and 0.79 (95% CI 0.54 to 1.14) for 90-day mortality. When the exposure of IHT was analysed in the multivariate model, it was associated with a lower risk of mortality for both 30 days (HR 0.47, 95% CI 0.30 to 0.76) and 90 days (HR 0.52, 95% CI 0.34 to 0.79) (table 2).
Supplemental material
Kaplan-Meier curve showing survival to day 30 from ICU admission for patients with SARS-CoV-2 infection in Sweden between 1 March 2020 and 30 June 2021. ICU, intensive care unit; IHT, interhospital transfer.
Kaplan-Meier curve showing survival to day 90 from ICU admission for patients with SARS-CoV-2 infection in Sweden between 1 March 2020 and 30 June 2021. ICU, intensive care unit; IHT, interhospital transfer.
Risk of death 30 and 90 days after IHT among critically ill patients with SARS-CoV-2 presented as HR with 95% CI (non-IHT as reference)
As the assumptions of proportional hazards were violated when scaled Schoenfeld residuals were regressed against survival time (p<0.001 for the multivariate model for 30-day and 90-day mortality), sensitivity analyses were performed. When splitting the time and analysing by different lengths of stay (LOS) at the ICU, the assumptions were fulfilled for patients with an LOS of 8 days and above (p=0.14 for 30-day mortality and p=0.09 for 90-day mortality). The results did not differ from the original models except for IHT being associated with lower 30-day mortality, including in the univariate analyses for patients with an ICU LOS of more than 6 and 8 days (table 3). Schoenfeld residuals indicated violations of proportional hazard assumptions, including for the covariates of mechanical ventilation, treatment limitations and COVID-19 wave in the multivariate models for 30 and 90 days. This issue was addressed by examining the various categories within these variables as subgroups. The reason for the violation of the variables of mechanical ventilation and treatment limitations was a small number of patients/events in the groups of patients without mechanical ventilation exposed to IHT and the group with treatment limitations exposed to IHT (see details in online supplemental files 2 and 3). When analysing the subgroup of only mechanically ventilated patients, the results changed in the univariate models, showing a lower risk of mortality for 30 days (19.3% vs 32.9%, p=0.008) and 90 days (26.0% vs 38.0%, p=0.029) in the IHT group compared with the non-IHT group. In the multivariate models, the decrease in mortality for the IHT group at 30 and 90 days remained unchanged. Excluding patients with treatment limitations did not change the results.
Supplemental material
Supplemental material
The sensitivity analysis presents the risk of 30-day and 90-day mortality following IHT, categorised by the length of ICU stay from admission to the day of transfer
Interhospital capacity transfer and mortality by COVID-19 wave
For the group of patients treated during the first COVID-19 wave, the univariate analyses for 30-day mortality indicated a reduction in deaths for the IHT group, with a HR of 0.26 (95% CI 0.10 to 0.73). The univariate analyses for 90-day mortality and the multivariate analyses for both 30-day and 90-day mortality remained consistent, with HRs of 0.44 (95% CI 0.20 to 0.96), 0.24 (95% CI 0.08 to 0.66) and 0.38 (95% CI 0.17 to 0.85), respectively (table 4).
The sensitivity analysis presents the risk of 30-day and 90-day mortality following IHT, categorised by pandemic waves
For the group treated during the second and third COVID-19 waves, the results differed from the original multivariate models. In this subgroup, there was still no significant difference between patients with and without IHT in the univariate models, with HRs of 0.95 (95% CI 0.58 to 1.54) for 30-day mortality and 1.01 (95% CI 0.65 to 1.54) for 90-day mortality. However, unlike the findings in the whole cohort, there was no longer a significant difference in the associated risk of 30-day and 90-day mortality in the multivariate models, with HRs of 0.68 (95% CI 0.38 to 1.18) and 0.63 (95% CI 0.38 to 1.04), respectively (table 4).
IHT and days alive free of ICU
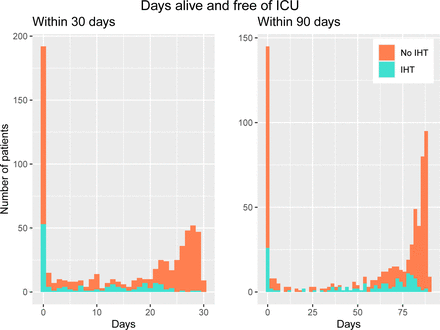
In unadjusted analyses, IHT patients had a median of fewer days alive and free from the ICU at 30 days compared with non-IHT patients (5, IQR: 0–18 vs 22, IQR: 0–27, p<0.001). At 90 days, there were no statistically significant differences (figure 4). In the subgroup of mechanically ventilated patients, no significant median differences were observed at either 30 or 90 days.
Days alive and free of ICU within 30 and 90 days among transferred and non-transferred patients. ICU, intensive care unit; IHT, interhospital transfer.
Discussion
In this study, transfers solely due to capacity constraints during the COVID-19 pandemic were not associated with a higher risk of 30-day and 90-day mortality among patients with critical COVID-19. Our results indicated that such transfers may even be associated with a lower mortality risk, especially for mechanically ventilated patients. IHT was associated with a longer ICU LOS compared with patients who remained in the admitting ICU. This was probably explained by the higher prevalence of invasive ventilation among transferred patients.
The risk of IHT has been investigated to some extent in observational studies, although with conflicting results. A Swedish register-based study of 2912 capacity-transferred ICU patients before the pandemic found that transportation was associated with a lower risk of death within 90 days (OR 0.71, 95% CI 0.65 to 0.79).19 Conversely, another Swedish study including 11 176 ICU patients showed a higher risk of 30-day mortality after capacity transfers (OR 1.25, 95% CI 1.06 to 1.49) and clinical transfers (OR 1.17, 95% CI 1.02 to 1.36).20 However, the reference consisted of repatriated patients. While we excluded such patients in our study, other studies have confirmed that clinical transfer to a higher level of care is associated with comparable patient outcomes.21 A French cohort study of 18 348 COVID-19 patients in the ICU found that transferred patients had a lower mortality rate than non-transferred patients, concluding that this might be due to a rigorous selection process of patients eligible for transfer.14 However, the study only included patients from the first wave of the pandemic. This finding resembles ours, where the results were driven by lower mortality in IHT patients during the first COVID-19 wave. A retrospective cohort study including 5207 patients mostly with SARS-CoV-2 during wave 3 of the pandemic in Australia found no association between IHT and mortality.11 The difference in outcomes between the pandemic waves may be attributed to changes in healthcare conditions among patients and the accumulated experience in managing the disease. Although capacity constraints remained high during the pandemic waves, the outcomes improved due to the learning curve in treating COVID-19 patients and the implementation of standardised treatments.
Combining the findings of the present study with the previous literature makes it reasonable to argue that IHT of ICU patients does not appear to increase mortality. Nevertheless, there may be other drawbacks to IHTs, such as hospital-acquired infections, discontinued care, information gaps or patients being intubated to ensure safe transport, which may prolong the hospital stay.22 23
The observed absence of increased mortality risk associated with the transfer may be explained by the fact that patients with recovery potential were carefully selected to be stable enough to tolerate transport safely. This aligns with the results from the above-mentioned French study.14 Furthermore, the technical difficulties associated with transporting patients with high-flow oxygen therapy with high oxygen fractions most likely contributed to the decision to avoid such transfers, which was reflected in our study where mechanical ventilation was more common in the transferred group.
The main methodological strengths of this study were the comprehensive data collection conducted by dedicated researchers during the three pandemic waves and validated by independent researchers, the low proportion of missing data and the adjustment for known confounders in the analyses. Among the main weaknesses are the single-centre design and the non-randomised design. There may be a selection bias in the study, with patients on invasive ventilation and therefore with a secured airway and having limitations of care to a lesser extent, such as do not resuscitate orders, in the transferred group. This difference might explain the lower OR for mortality in the adjusted analysis and also the longer ICU LOS, as the treatments were not discontinued. In this study, we selected mortality and days alive and out of the ICU as one of the outcome measures. However, we acknowledge that organ support-free days could have been another valuable parameter to assess the impact of transfers. This alternative measure could provide additional insights into the safety of IHTs. Assessing illness during transport and comparing different patient groups presents several challenges. Information about the severity of illness immediately before transport, which might be more appropriate to include in the analyses than the severity of illness at ICU admission, was not readily available. We did not find sufficient and reliable data to analyse this in our cohort. Furthermore, we used SAPS III to assess illness severity instead of the more commonly used systems like Acute Physiology and Chronic Health Evaluation II or IV (APACHE II or IV). SAPS III is the standard scoring system for intensive care patients in Sweden and is used for reporting to the Swedish Intensive Care Register, ensuring consistency and accuracy within the national context. However, this choice may limit the direct comparability of our results with studies using other scoring systems. 14% of SAPS data were missing. The missingness was primarily due to manual documentation processes during the pandemic, leading to a gap at one of the ICUs. This gap was likely caused by work-related issues rather than patient-related factors. Patients across the two ICUs were similar in clinical characteristics and diagnoses, and all other variables were complete, supporting the assumption that the missing data were random. We also conducted a sensitivity analysis with complete cases, and the results remained unchanged. Another issue was that lifestyle factors such as smoking, physical activity and alcohol consumption, as well as socio-economic variables like deprivation, education and employment status, were not available in our dataset. As such, we were unable to adjust for these variables in the analysis. Furthermore, we have not investigated patients’ functional status after ICU discharge, which is of high importance for evaluating surviving patients’ well-being.
Differences in mortality between countries may depend on variations in healthcare structure and the degree of burden that the country and the healthcare organisation experienced during the pandemic. Well-established routines for transport, availability of advanced medical transport equipment and access to the same electronic patient data management systems for transferring and admitting centres may also influence the safety of transport. The findings of this study may be somewhat representative of countries with comparable population structures, healthcare systems and pandemic responses. The use of standardised healthcare protocols ensures consistency in patient care and treatment, which can make the results of this study applicable to other regions facing similar challenges. Future studies should focus on whether certain patient characteristics are predictive of safe transfers and should study the effects of transfers on surviving patients’ ability to recover. Furthermore, it is important to explore various types of complications related to IHT and long-term outcomes to identify factors that may influence treatment outcomes and thereby improve care.
Conclusions
In our study, IHT due to capacity constraints among critically ill COVID-19 patients was not associated with a higher mortality risk. The suitability for transfer was likely associated with lower mortality, although residual confounding cannot be ruled out. The requirement for invasive ventilation among transferred patients might account for the extended length of ICU stay, rather than the transfer itself. However, the difficulty in studying this issue lies in the fact that while patients are likely exposed to risks during transfer, they are simultaneously the patients stable enough to be transported.
Data availability statement
Data are available on reasonable request. The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the Swedish Ethical Review Authority (Dnr: 2023-01778-01-380347, amendment 2023-01778-01). This was a register based study. The requirement for written informed consent from patients was waived.
Acknowledgments
Special thanks to MD Jacob Litorell for developing, validating and maintaining the COVID-19 database we used in this study.
Footnotes
LTA and KB are joint first authors.
Contributors KB and ARS: Conceptualisation, methodology, design, analysis, writing the original draft, review and editing. LTA: Conceptualisation, methodology, design, writing the original draft, review and editing. PS, MC and EJ-A: Conceptualisation, methodology, design, review and editing. SJ: Conceptualisation, methodology, design, analysis, review and editing. ARS: Responsible for the overall content as guarantor.
Funding The study was supported by grants from the Swedish patient insurance company (LÖF). The funders had no role in the design or conduct of the study.
Competing interests None declared.
Patient and public involvement statement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.