Article Text
Abstract
Objectives Herpes zoster (HZ) infection is associated with a higher risk of major adverse cardiovascular events (MACE), including stroke and coronary artery disease (CAD). Patients with diabetes are at an increased risk of MACE, highlighting the importance of studying this population to assess the potential protective effects of HZ vaccination. This study aims to investigate the risk of MACE after HZ vaccination in patients with diabetes.
Design Retrospective cohort study.
Setting Community-based population in the USA.
Participants Using the TriNetX database, the study included 4.9 million patients with diabetes from 2006 to 2022. It established two cohorts: 68 178 patients in the HZ vaccination (comprising any HZ vaccine, Shingrix or Zostavax) and 4 835 246 patients in the no HZ vaccination group. After excluding patients with a history of MACE, immune disease and complications of HZ prior to the index date, the study cohort was reduced to 45 960 patients. Propensity score matching, accounting for age, sex, race, socio-economic status and disease comorbidities, was conducted to minimise study bias.
Interventions HZ vaccination.
Outcome measures MACE outcomes are defined as the first occurrence of CAD or stroke. Comparative risk analysis was conducted using HRs.
Results Post matching, the mean patient age was 63.5 years, with 49.2% females. The incidence rate of MACE was lower among vaccinated patients compared with unvaccinated individuals, with an HR of 0.76 (0.72–0.79). For secondary endpoints, the HRs were 0.73 (0.69–0.78) for CAD, 0.79 (0.74–0.84) for stroke and 0.54 (0.52–0.57) for all-cause mortality. These protective effects remained consistent across different age groups, sexes and diabetes types, supporting the potential benefit of HZ vaccination in reducing cardiovascular risk.
Conclusions HZ vaccination is associated with a lower risk of MACE in patients with diabetes. Further prospective studies are critically needed to confirm this finding.
- INTERNAL MEDICINE
- Vaccination
- DIABETES & ENDOCRINOLOGY
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study used a large community-based database, providing robust and representative data for analysis.
This study includes a long follow-up duration, allowing us to assess the impact of herpes zoster vaccination on major adverse cardiovascular events (MACE) risk over an extended period.
This study evaluated the risk of MACE after herpes zoster vaccination in patients with diabetes.
This study is limited by the potential for unmeasured confounding that cannot be entirely eliminated.
Introduction
Herpes zoster (HZ), commonly known as shingles, is a prevalent viral infection caused by the reactivation of the varicella zoster virus, which remains latent in the body following an initial chickenpox infection.1 Triggered typically by ageing, immunosuppression or stress, this reactivation manifests as painful, blistering skin eruptions localised to specific dermatomes.2 3 Additionally, It is particularly noted for its complications, such as postherpetic neuralgia, which can cause prolonged discomfort.4 5 Recent studies have shifted focus towards the broader impacts of HZ, especially its association with an increased risk of major adverse cardiovascular events (MACE), including stroke and myocardial infarction.6–18 Importantly, research suggests that the risk of stroke is time-dependent following an HZ infection, with a significant elevation in the first month at 78%, reducing to 43% after 3 months and further to 20% after 1 year, before levelling off to a non-significant 7% increase up to 3 years post infection.19 This time-dependent risk profile underscores the importance of timely intervention and prevention strategies.
Within the population of individuals with diabetes mellitus, the interplay between HZ infection and cardiovascular risk is of particular concern. Diabetes, a chronic condition characterised by elevated blood glucose levels, significantly heightens the risk of cardiovascular diseases, making this group particularly susceptible to the compounded effects of HZ infection.19 20 The risk of cardiovascular events in patients with diabetes is two- to threefold higher than in those without diabetes, underscoring the critical need for comprehensive strategies to mitigate these risks.21 22 The exacerbation of cardiovascular complications by HZ may be mediated through vasculopathy, a process potentially involving direct viral invasion of intra- or extracranial arteries, culminating in vessel wall damage through inflammatory responses characterised by multinucleated giant cells and epithelioid macrophages.23–27 Additionally, HZ may provoke an inflammatory environment within the vessel wall, fostering a pro-coagulation state, further underscoring the complex interrelation between HZ infection and cardiovascular morbidity in diabetes.24 28 29
The advent of HZ vaccines, such as the recombinant zoster vaccine (or Shingrix) and the live-attenuated zoster vaccine (LZV or Zostavax), offers a promising strategy for reducing the incidence of HZ and its associated complications.30–32 These vaccines have demonstrated robust efficacy in the general population aged 50 years and older, reducing both the occurrence of HZ and the severity of postherpetic neuralgia.33 Given the established link between HZ infection and an increased risk of cardiovascular events, it is plausible to hypothesise that HZ vaccination could also confer protective effects against MACE, particularly in the diabetes population. However, prior research investigating the relationship between HZ vaccination and cardiovascular events has yielded mixed outcomes. Specifically, Parameswaran and colleagues,34 using Veteran Affairs data, observed a significant protective effect against stroke in elderly male vaccine recipients (both Zostavax and Shingrix). Their study noted that patients faced a higher stroke risk within the first month following recent HZ infection, but individuals who received at least one zoster vaccination demonstrated a mitigation of this elevated risk, with ORs of 0.57 (95% CI: 0.46 to 0.72) for Shingrix and 0.77 (95% CI: 0.65 to 0.91) for Zostavax at 30 days post event. In contrast, Minnasian et al,35 using Medicare data from individuals older than 65 years, identified a transiently heightened risk of stroke and myocardial infarction associated with HZ infection—most pronounced within the first week following zoster diagnosis—yet did not detect a reduction in the incidence of these events in HZ vaccine recipients within the initial 4 weeks post infection. Yang et al,36 37 in separate analyses of the US Medicare population, found a 16% reduction in stroke risk among vaccine recipients aged 66 and older, with enhanced benefits noted in specific subgroups. These varying findings may stem from differences in population demographics (eg, age ranges, underlying comorbidities) and follow-up durations (eg, short-term vs long-term surveillance). Despite these efforts, it remains unclear whether HZ vaccination consistently confers a true protective effect, particularly among high-risk individuals such as those with diabetes, where the burden of cardiovascular disease is already elevated. Thus, a critical gap remains in establishing whether HZ vaccination offers meaningful cardiovascular benefits in patients with diabetes, underscoring the need for more targeted research in this domain.
Methods
Study population
This retrospective cohort study used data from the TriNetX database, which aggregates electronic medical records from healthcare organisations across the USA. The TriNetX database is a comprehensive repository of deidentified electronic health records from a diverse range of healthcare organisations, including hospitals, clinics and medical practices. It encompasses data on patient demographics, diagnoses, procedures, medications, laboratory results and other clinical variables. The TriNetX database has been validated and widely used in many representative publications, supporting its credibility for research purposes.38–40 The total number of patients available in the TriNetX network is 112 million.
Cohort selection
Cases were defined as individuals with ages 50 or older, diagnosed with diabetes mellitus, who received HZ vaccination, including Shingrix or Zostavax, within 1 year of their diabetes diagnosis, with the index date set as the date of vaccination. This timeframe was chosen to minimise potential differences and biases between cases and controls. Conversely, the control group comprised patients with diabetes who did not receive any HZ vaccination during the study period, with the index date corresponding to the first date of diabetes diagnosis. This study was conducted from 1 January 2006 to 12 December 2022.
Exclusion criteria
Patients with a history of MACE before the index date were excluded to ensure that the study focused on incident cases of cardiovascular events rather than pre-existing conditions. Immunocompromised individuals were excluded because their underlying conditions might confound the relationship between HZ vaccination and MACE. These conditions, such as HIV, malignancy and immune diseases (rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis), can affect the immune response and potentially influence the risk of cardiovascular events. Excluding individuals with a prior diagnosis of HZ and its complications (postherpetic neuralgia, Bell’s palsy, Ramsay-Hunt syndrome) before the index date helped ensure that only new cases of these conditions were considered during the study period, reducing potential bias in the analysis.
Study codes and disease comorbidities
Study codes and disease comorbidities are detailed in online supplemental table 1. In summary, the coding for diabetes diagnosis used the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10 CM) codes of E10-E11, while patients who received HZ vaccination were identified with procedure code and medical prescription normalised medical prescription (RxNorm). Furthermore, disease comorbidities such as hypertension, obesity and chronic kidney disease (CKD) were allocated specific codes for identification and analysis purposes. This comprehensive coding system facilitated the organisation and interpretation of patient data, ensuring clarity and precision in the study’s findings. The definition of socio-economic status (SES) in our study is based on ICD-10 coding (Z55-Z65), which includes factors related to education, employment, income, and social environment.
Supplemental material
This study aimed to investigate the association between HZ vaccination and the incidence of MACE among individuals with diabetes aged 50 years and older. The focus on this age group was driven by their heightened risk of MACE and their alignment with vaccination guidelines.41 The primary endpoint of this study is defined as the first occurrence of composite MACE, comprising coronary artery disease (CAD) or stroke following the index date. Secondary endpoints include individual outcomes of CAD, stroke and all-cause mortality. Subgroup analysis was conducted by stratifying age, sex and type of diabetes. Additionally, we explored the risk of MACE within the first year of follow-up.
Propensity score matching
Propensity score matching (PSM) is a statistical technique used to balance cohorts in observational studies by adjusting for potential confounders. It ensures comparability between the HZ vaccine and no HZ vaccine groups when randomisation is not feasible. This is achieved by estimating the probability, or ‘propensity score’, of a patient belonging to one cohort based on observed covariates.
In this study, researchers defined two cohorts of interest (HZ vaccine vs no HZ vaccine) and identified covariates—factors that may influence both treatment allocation and outcomes. These covariates include age, sex, race, SES and various comorbidities, such as hypertensive diseases, overweight and obesity, other forms of heart disease, CKD, neoplasms, nicotine dependence, hypertensive CKD, alcohol-related disorders, fibrosis and cirrhosis of the liver, unspecified dementia, alcoholic liver disease, Alzheimer’s disease, dementia, hepatic failure, chronic hepatitis, vascular dementia and rheumatoid arthritis with rheumatoid factor.
Using logistic regression, the system calculates each patient’s propensity score, which reflects the probability of belonging to a specific cohort given the covariates. The system employs a greedy nearest-neighbour matching with a calliper of 0.1 pooled SD, ensuring that patients in the smaller cohort are matched with those in the larger cohort based on the closest propensity scores within the defined range. This process generates balanced matched subsets.
After matching, the outcomes of interest are compared between these balanced subsets rather than the original cohorts, effectively minimising the effects of confounding variables. PSM is implemented within a federated data network, pooling data from multiple healthcare organisations. To mitigate bias introduced by the order of data during matching, patient records are randomised prior to matching. Deterministic randomisation is applied to ensure the reproducibility of the analyses. The PSM analysis for this study was conducted using the built-in tools provided by the TriNetX platform.
To evaluate the impact of HZ vaccination on MACE, we divided the study into four populations for analysis, designated as models 1–4. The matching process involved four different comparisons: (1) cases vaccinated with any HZ vaccine matched to no-HZ-vaccinated controls (model 1), (2) cases vaccinated with Shingrix matched to no-HZ-vaccinated controls (model 2), (3) cases vaccinated with Zostavax matched to no-HZ-vaccinated controls (model 3) and (4) cases vaccinated with Shingrix matched against those vaccinated with Zostavax (model 4). This approach allowed us to assess both the overall effect of HZ vaccination and direct comparisons between vaccine types.
Statistical analysis
TriNetX ensures data quality through rigorous checks and monitoring. The platform validates data formatting, ensuring proper representation of dates and required fields (eg, patient identifiers), rejecting records with missing essential information. Referential integrity checks verify successful data integration across tables, while volume trends are monitored during data refreshes to maintain validity. Patient records must include at least one non-demographic fact to be included as records with only demographic data are excluded. TriNetX collaborates with data providers by sharing regular feedback and data quality scorecards, enabling providers to assess their data quality and compare it with peers based on regional or population-specific benchmarks. Data quality is assessed at various stages: during onboarding of new providers, periodic data refreshes, significant pipeline changes or troubleshooting requests. The process is dynamic, with ongoing improvements in metrics, collection methods and evaluation procedures to enhance overall data reliability and operational efficiency.
TriNetX ensures cohort integrity by using a master patient index, tokenisation and data normalisation to prevent duplicate patient records. It applies cross-site deduplication, distinct patient count algorithms and real-time filtering to ensure each patient is counted only once, minimising bias and maintaining data accuracy in research analyses.
Descriptive statistics were employed to summarise the baseline characteristics of the study population, including age, sex, race, SES and disease comorbidities. Following PSM, the balance between matched cohorts was evaluated using standardised mean differences (SMD), where an SMD value of less than 0.1 was considered indicative of a well-matched cohort. The incidence of MACE was analysed using a Kaplan-Meier survival curve with statistical significance determined using the log-rank test. A Cox proportional hazards model was further applied to evaluate the association between group assignment and the risk of MACE and all-cause mortality, providing HRs with 95% CIs. All analyses were performed using the TriNetX online platform, which uses R V.4.0.2 as its underlying statistical framework.
Sensitivity analysis
To address potential healthy vaccine bias, we conducted a post hoc sensitivity analysis by identifying a subgroup of patients who received HZ vaccination at least 1 year after their diabetes diagnosis. This additional analysis aimed to determine whether delaying vaccination after diabetes diagnosis affected the primary outcomes.
Patient and public involvement
None.
Results
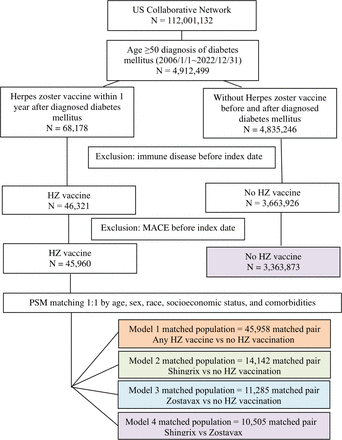
This study included a total of 112 million patients (figure 1). Following the filtration process to identify patients with a diagnosis of diabetes, we narrowed the cohort down to 4.9 million patients. Among these, 68 178 patients were identified as cases, having received any HZ vaccination within 1 year of diagnosis of diabetes, while 4 835 246 patients served as controls, having diabetes without any HZ vaccination. Further exclusion of patients with immune diseases and a history of MACE before the index date resulted in 45 960 cases for any HZ vaccination and 3 363 873 controls. Subsequently, we divided the study into four populations for evaluation, designated as models 1–4. The matching of cases vaccinated with any HZ vaccine to no-HZ-vaccinated controls yielded 45 958 pairs (model 1). Meanwhile, matching cases vaccinated with Shingrix to no-HZ-vaccinated controls resulted in 14 142 pairs (model 2), and matching cases vaccinated with Zostavax to no-HZ-vaccinated controls resulted in 11 285 pairs (model 3). Finally, matching cases vaccinated with Shingrix against those vaccinated with Zostavax resulted in 10 505 pairs (model 4).
Detailed flowchart illustrating the division of participants into four groups based on herpes zoster (HZ) vaccination status. Matching of any HZ-vaccinated cases to no-vaccinated controls yielded 45 958 pairs (model 1). Matching Shingrix-vaccinated to no-vaccinated controls resulted in 14 142 pairs (model 2), Zostavax-vaccinated to no-vaccinated controls yielded 11 285 pairs (model 3) and Shingrix vs Zostavax vaccination resulted in 10 505 pairs (model 4). MAZE, major adverse cardiovascular events; PSM, propensity score matching.
Table 1 presents the baseline characteristics for both HZ vaccination cases and no HZ vaccination controls. Prior to PSM, notable differences were observed in several comorbidities, including hypertensive diseases, obesity, heart disease, CKD, neoplasm and nicotine dependence. The mean age was 63.5 years, with 49.1% female and 58.9% white race. Disease comorbidities included patients with hypertensive disease accounting for 54.8%, overweight and obesity at 19.5%, other forms of heart disease at 12.3%, CKD at 7.1%, neoplasm at 8.3% and nicotine dependence at 5.8%. Patients with SES issues accounted for 1.1% of the HZ vaccination group. Following the matching process, the disparity between cases and controls was significantly reduced, as evidenced by the SMD being less than 0.1, detailed in online supplemental tables 2–5.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Demographic characteristics of unmatched individuals vaccinated versus unvaccinated against HZ
Table 2 presents the risk of MACE among patients with HZ vaccination compared with those without vaccination. The risk of MACE, CAD, stroke and all-cause mortality was consistently lower among patients with any HZ vaccination compared with those without vaccination, as evidenced byHRs and 95% CIs of 0.76 (0.72 to 0.79), 0.73 (0.69 to 0.78), 0.79 (0.74 to 0.84) and 0.54 (0.52 to 0.57), respectively. These findings underscore the potential protective effect of any HZ vaccination against adverse cardiovascular outcomes. When used individually, both Shingrix and Zostavax demonstrated effectiveness in reducing the risk of MACE, CAD, stroke and all-cause mortality compared with no vaccination. For Shingrix, the risks were 0.84 (0.76 to 0.91) for MACE, 0.78 (0.69 to 0.88) for CAD, 0.87 (0.77 to 0.99) for stroke and 0.53 (0.48 to 0.58) for all-cause mortality. Similarly, Zostavax showed HR and 95% CI of 0.81 (0.75 to 0.88) for MACE, 0.72 (0.65 to 0.80) for CAD, 0.90 (0.81 to 1.01) for stroke and 0.58 (0.53 to 0.62) for all-cause mortality.
Risk of MACE among patients receiving HZ vaccination compared with no vaccination and head-to-head comparison of Shingrix versus Zostavax
These results suggest that both Shingrix and Zostavax offer protective benefits against MACE when administered individually. When comparing Shingrix with Zostavax, interesting findings emerged. While a neutral result was observed for MACE and stroke, a notable difference was detected in CAD. The HR and 95% CI for CAD were 1.16 (1.01 to 1.34), indicating a higher risk of CAD among individuals receiving Shingrix compared with Zostavax. However, no significant differences were noted in stroke, all-cause mortality or overall MACE between the two vaccines. This highlights the importance of considering specific cardiovascular outcomes when evaluating the comparative effectiveness of different HZ vaccines.
The stratification analysis of the risk of MACE among different groups revealed consistent findings across various demographic and clinical factors (table 3). Regardless of age, individuals aged 50–65 years and those over 65 years demonstrated a lower risk of MACE with HZ vaccination compared with no vaccination, with HR and 95% CI of 0.80 (0.75 to 0.86) and 0.83 (0.78 to 0.89), respectively. Similarly, both females and males experienced a reduced risk of MACE with vaccination, with HR and 95% CI of 0.77 (0.72 to 0.83) and 0.74 (0.69 to 0.79), respectively. Furthermore, individuals with type 1 or 2 diabetes also exhibited a lower risk of MACE with HZ vaccination compared with no vaccination, with HR and 95% CI of 0.25 (0.08 to 0.75) for type 1 diabetes and 0.71 (0.68 to 0.75) for type 2 diabetes. These consistent protective effects across different age groups, sexes and types of diabetes underscore the robustness of the association between HZ vaccination and reduced cardiovascular risk.
Stratification analysis of risk of major adverse cardiovascular events among different group in the model 1 matched population
When considering the timing within the first year of vaccination, table 4 illustrates a notable trend in the risk of MACE. The risk of MACE is observed to be the lowest in the first month following vaccination, with an HR and 95% CI of 0.21 (0.16 to 0.27). Subsequently, the risk of MACE gradually increases over time, yet remains significantly lower compared with no vaccination. At the end of the first year, the HR and 95% CI for MACE stand at 0.57 (0.52 to 0.62). In the long-term follow-up, as depicted in online supplemental table 6, the risk of MACE demonstrates consistent patterns across different time intervals. Over a follow-up period of up to 5 years, individuals with HZ vaccination exhibit a significantly lower risk of MACE compared with unvaccinated counterparts, with an HR and 95% CI of 0.70 (0.66 to 0.74). However, the protective effects seem to wane with time. During follow-up periods of 5–10 years and beyond 10 years, the HR and 95% CI for MACE among vaccinated individuals are observed to be 0.93 (0.84 to 1.02) and 1.13 (0.92 to 1.39), respectively.
Supplemental material
Risk of major adverse cardiovascular events within a 1-year follow-up period in the model 1 matched population
The protective efficacy of Shingrix demonstrates consistency, whether administered as a single dose or a two-dose regimen, compared with a no-HZ-vaccinated control group. Specifically, the HR for individuals receiving one dose of Shingrix was 0.66 (95% CI: 0.59 to 0.73), while for those completing the two-dose regimen, the HR was 0.73 (95% CI: 0.59 to 0.89), as detailed in online supplemental table 7. Furthermore, a post hoc sensitivity analysis was conducted by identifying a subgroup of patients who received HZ vaccination at least 1 year after their diabetes diagnosis. The results were consistent with our primary findings, confirming that the protective effect of HZ vaccination against MACE remained robust, regardless of the timing of vaccination relative to diabetes diagnosis (online supplemental figure S1). Detailed results of this analysis are provided in online supplemental tables 8 and 9.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
The Kaplan-Meier survival curve (online supplemental figure 2) illustrates the cumulative incidence of MACE over time, comparing HZ vaccinated versus unvaccinated patients and a head-to-head analysis of Shingrix versus Zostavax. The curves show a lower cumulative incidence of MACE in vaccinated patients, suggesting a protective effect of HZ vaccination. In the Shingrix versus Zostavax comparison, the results indicate a neutral effect between the two vaccines, with no significant difference in MACE risk.
Supplemental material
Discussion
To the best of our knowledge, this study represents the first comprehensive investigation into the risk of MACE among patients with diabetes following HZ vaccination. Our findings reveal a significant decrease in the risk of MACE subsequent to HZ vaccination. This protective effect extends to other critical outcomes, including CAD, stroke and all-cause mortality, demonstrating consistent benefits across multiple cardiovascular endpoints. Furthermore, our subgroup analysis highlights the robustness of the protective effect as it remains consistent across different age groups, sexes and types of diabetes. Interestingly, our study also indicates that the strongest protective effects appear to manifest within the first year following vaccination, but these effects appear to diminish over time. These findings underscore the potential additional benefits of HZ vaccination in reducing cardiovascular risk among individuals with diabetes.
HZ is increasingly being investigated for its potential link to cardiovascular disease. Initial evidence suggesting HZ as a risk factor for cardiovascular disease comes primarily from retrospective analyses,6–18 which have documented a higher frequency of cardiovascular events—such as stroke and myocardial infarction—in individuals who have had HZ episodes compared with those who have not. Following these preliminary observations, further research aimed at confirming and expanding on this association has been conducted through larger-scale studies across diverse global populations. This extensive research has shown an increased risk of cardiovascular events post-HZ infection, underscoring the necessity for increased clinical awareness and management of cardiovascular risk factors among those with a history of HZ.34 37
Several mechanisms have been proposed to elucidate the link between HZ infection and an increased risk of MACE. A primary mechanism believed to be implicated is vasculopathy, wherein the virus directly infects and spreads from the nerve to the cerebral artery, eliciting inflammation, pathological vascular remodelling and subsequently heightening the risk of stroke.25 42 Moreover, beyond the direct vascular effects, HZ infection may contribute to elevated blood pressure due to the pain and stress associated with the condition. This elevation in blood pressure could further exacerbate the risk of stroke, given that hypertension is a leading cause of stroke.
Within the existing literature, our study stands out for evaluating patients with the longest follow-up duration and focusing specifically on the diabetes population. Notably, three published studies have been identified, each presenting unique findings. Parameswaran et al34 and Yang et al37 reported positive HZ vaccination outcomes, while Minnasian et al35 found no significant advantage. These studies, characterised by retrospective designs, differ in their data sources, study populations and methodologies, contributing to the heterogeneity in results.
The distinctive aspect of our study lies in the examination of patients aged between 50 and 65 years old, a demographic often under-represented in similar analyses.34 35 37 This age group, typically considered lower risk for MACE compared with those over 65, exhibited intriguing results in our study. Specifically, we observed a significantly reduced risk of MACE among patients with diabetes aged 50–65 who received HZ vaccination, with an HR of 0.80 (95% CI: 0.75 to 0.86), compared with unvaccinated counterparts. This finding provides valuable insights into the effectiveness of HZ vaccination in reducing MACE risk among individuals who might benefit most from early preventive measures. Another unique aspect of our study is the inclusion of data on patients with type 1 diabetes who received HZ vaccination, a demographic that has been largely overlooked in previous literature. To our knowledge, this is the first study to report outcomes for individuals with type 1 diabetes following HZ vaccination. Our analysis revealed a noteworthy finding, indicating a significantly reduced risk of MACE among patients with type 1 diabetes who received HZ vaccination, with an HR of 0.25 (95% CI: 0.08 to 0.75). This novel insight underscores the potential benefits of HZ vaccination not only for individuals with type 2 diabetes but also for those with type 1 diabetes, highlighting the importance of considering this population in future vaccination strategies and guidelines.
Parameswaran and colleagues, using Veteran Affairs data, observed a significant protective effect against stroke in elderly males following vaccination with both Zostavax and Shingrix.34 Their study revealed that patients experienced a notably higher risk of stroke within the first month following recent HZ infection. However, individuals who received at least one zoster vaccination demonstrated a mitigation of this increased risk. Specifically, the OR for stroke 30 days post event was 0.57 (95% CI: 0.46 to 0.72) for Shingrix and 0.77 (95% CI: 0.65 to 0.91) for Zostavax. Similarly, Yang et al, analysing US Medicare data, identified a 16% reduction in stroke risk among vaccine recipients aged 66 and older, with enhanced benefits observed in specific subgroups.37
Minnasian et al’s study,35 conducted within the Medicare population and focusing on patients older than 65 years, revealed a transiently heightened risk of stroke and myocardial infarction associated with HZ infection. Particularly noteworthy was the pronounced increase observed within the initial week following zoster diagnosis, with a 2.4-fold elevated rate of ischaemic stroke (incidence rate (IR) 2.37, 95% CI: 2.17 to 2.59) and a 1.7-fold increase in myocardial infarction rate (IR 1.68, 95% CI: 1.47 to 1.92), followed by a gradual reduction over 6 months. However, the study did not find evidence of a reduction in the IR for ischaemic stroke or myocardial infarction among HZ vaccine recipients in the first four weeks following zoster diagnosis. The lack of observed protective effects of the HZ vaccine may be attributed to the limited number of patients in the vaccinated groups, thereby restricting the study’s power to adequately assess this outcome. Notably, only 9% of participants received the vaccine during the study period, underscoring the challenge of assessing vaccine effectiveness in real-world settings with low uptake rates. These disparities underscore the importance of considering study-specific factors, such as data sources and population characteristics, when interpreting and comparing research findings.
An additional significant discovery from our research is the most robust protective impact of HZ vaccination against MACE observed during the first year, with this protective effect extending over 5 years of follow-up. This outcome aligns with the observation that the highest risk of stroke occurs within the first year.19 This phenomenon could be attributed to various potential mechanisms. First, the vaccine may modulate the immune response, reducing systemic inflammation, a key contributor to atherosclerosis and cardiovascular events. Furthermore, by preventing HZ, the vaccine indirectly decreases cardiovascular stress, considering the association between HZ and a heightened risk of stroke and myocardial infarction, particularly in the first year following infection. This dual mechanism—lowering inflammation and averting HZ—accounts for the observed sustained, although gradually decreasing, protective effect over time.
The discrepancy between the sum of population models 2 and 3 not equaling the total of model 1 can be attributed to the specific inclusion criteria based on procedural and medication codes used to identify the vaccination status within our study cohorts. Model 1 encompasses a broader category of individuals vaccinated with any HZ vaccine, identified through a comprehensive set of codes, including CPT codes 90736 (Zostavax) and 90750 (Shingrix), as well as additional codes for unspecified zoster vaccines (459891000124012) and their respective RXNORM codes (1292422 for Zostavax and 1986821 for Shingrix). This allows for the inclusion of all individuals vaccinated against HZ, capturing a wider demographic. Conversely, models 2 and 3 focus on narrower subsets, with model 2 including only those vaccinated with Shingrix (via CPT code 90750 and RXNORM code 1986821) and model 3 comprising individuals vaccinated with Zostavax (identified by CPT code 90736 and RXNORM code 1292422).
Observing a greater number of events in the Zostavax vaccination group compared with the control group, while the HR remains less than 1, highlights the nuanced nature of HR as a measure of relative risk over time rather than a simple count of events (table 2). This phenomenon indicates that, after adjusting for the duration of follow-up and baseline risk factors, individuals in the Zostavax group experienced a lower rate of events at any given time compared with the no-vaccinated group. The HR less than 1 suggests a protective effect of the Zostavax vaccine, reflecting its efficacy in reducing the instantaneous risk of adverse outcomes, despite the apparent higher number of events when viewed without the context of time and population size adjustments. This underscores the importance of HR in providing a more accurate assessment of the vaccine’s impact on health outcomes.
It is important to note that the discrepancies in total numbers between tables 2 and 3, as well as in other subgroups, are caused by the methodology employed in the TriNetX analyses. Each stratified analysis involves rematching individuals based on specific criteria, leading to variations in sample sizes and the number of participants experiencing MACE across different tables or subgroups. This rematching process is designed to ensure that comparisons within each stratification are appropriate and accurate, taking into account the varying characteristics of participants within each subgroup. Consequently, the figures for the total number of individuals and those experiencing MACE in one table cannot simply be summed to match the figures in another table due to these inherent differences in sample composition and size resulting from the rematching process.
An intriguing finding emerged from our study when directly comparing the effectiveness of Shingrix and Zostavax as there is a notable scarcity of head-to-head comparisons in the existing literature, particularly regarding their impact on MACE outcomes. Interestingly, while the American Diabetes Association (ADA) recommends Shingrix vaccination for individuals aged 50 years and older with diabetes,41 our study observed comparable outcomes between Zostavax and Shingrix, with a slight difference in CAD risk favouring Zostavax. However, it is imperative to interpret these findings with caution as our analysis is retrospective in nature and there exists a marked difference in the study timing between Zostavax and Shingrix. The reasons for this discrepancy are not fully elucidated but may relate to differences in vaccine composition and the resulting immune response. Zostavax, being a live-attenuated vaccine, could potentially elicit a broader and more robust immune response compared with Shingrix, which is a recombinant subunit vaccine. Moreover, Zostavax offers the convenience of requiring only one injection for full protection, whereas Shingrix necessitates two injections. The variations in the immune response elicited by these vaccines may contribute to differences in their effectiveness in preventing MACE outcomes among individuals with diabetes.
Our study benefits from several strengths that enhance the reliability and significance of our findings. First, leveraging data from the TriNetX database, which aggregates electronic medical records from 61 healthcare organisations across the USA, provided a robust and extensive data set for analysis. Second, employing a rigorous retrospective cohort study design enabled us to investigate the association between HZ vaccination and MACE among individuals with diabetes with clarity and precision. Additionally, our detailed analysis, including comprehensive stratification by age, sex and diabetes type, allowed for a nuanced understanding of vaccine effectiveness across diverse subgroups. Lastly, our study’s long-term follow-up, assessing MACE outcomes over up to 10 years post vaccination, provides valuable insights into the enduring protection offered by HZ vaccination against cardiovascular events.
Despite its strengths, our study is not without limitations. First, despite efforts to control for confounding variables, the potential for residual confounding cannot be entirely eliminated. Variables such as lifestyle factors, medication adherence and unmeasured comorbidities may contribute to unmeasured confounding. Second, the generalisability of our findings may be restricted due to the reliance on data from a single database comprising healthcare organisations solely within the USA. Lastly, the retrospective nature of our study design precludes the establishment of causal relationships between HZ vaccination and MACE, warranting cautious interpretation of our results and emphasising the need for further prospective investigations.
Further prospective studies are crucial to comprehensively evaluate the effectiveness of HZ vaccination in individuals with diabetes. Such prospective research should aim to assess vaccination outcomes in patients with diabetes across various time intervals following vaccination, allowing for a comprehensive understanding of the long-term efficacy and safety profiles of different vaccines, including Shingrix and Zostavax. By conducting such studies, researchers can address existing gaps in the literature and provide more definitive evidence to guide clinical decision-making and vaccination strategies in this vulnerable population.
In conclusion, our retrospective cohort study provides valuable insights into the association between HZ vaccination and MACE among individuals with diabetes. Despite the inherent limitations of retrospective analyses, our findings suggest a potential protective effect of HZ vaccination against MACE, aligning with the ADA recommendation to vaccinate individuals aged 50 and older with diabetes against HZ. Our study underscores the importance of HZ vaccination as a potential strategy for reducing cardiovascular risk in this vulnerable population. Moreover, beyond its known benefits in reducing the risk of HZ, our findings suggest that HZ vaccination may also contribute to lowering the risk of MACE.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The patient data used in this study were fully deidentified to ensure privacy and confidentiality. This procedure was implemented to prevent the direct or indirect identification of individual patients, thereby safeguarding patient privacy in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations. The study protocol was approved by the Institutional Review Board of Chung Shan Medical University Hospital, identified by the reference number CS2-23159.
Acknowledgments
Special thanks for Jing-Yang Huang and all study team for their dedication and support in this study.
References
Footnotes
Contributors EK: guarantor, conceptualisation, methodology, writing—original draft. S-CL: conceptualisation, methodology. C-NH: resources, supervision, data analysis. C-CW: investigation, review and editing. Y-HW: data curation, investigation, software, visualisation, data analysis. Y-SY: methodology, data analysis, writing—review and editing.
Funding This work was supported by grants from the Chung Shan Medical University Hospital (CSH-2022-A-022 and CSH-2025-A-029).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.