Article Text
Abstract
Background Osteonecrosis of the femoral head (ONFH) is characterised by progressive bone death, leading to joint incongruity and eventual osteoarthritis. Various interventions have been explored to forestall disease progression and delay total hip arthroplasty (THA). Free vascularised fibula grafting (FVFG) has shown promise, particularly in the precollapse stages of ONFH. However, the potential benefits of combining synovectomy with FVFG to address synovitis in ONFH have not been systematically studied. This trial seeks to compare outcomes between patients undergoing FVFG with and without synovectomy.
Methods and analysis The trial is a randomised, single-centre, parallel-group trial comparing FVFG with synovectomy versus FVFG alone in patients with ONFH and synovitis. 90 participants will be randomised into two groups: synovectomy (n=45) and non-synovectomy (n=45). The primary outcome is the Harris Hip Score (HHS) change at 12 months post surgery. Secondary outcomes include HHS, Numeric Rating Scale (NRS) for pain, Depression Anxiety Stress Scales-21 (DASS-21), and EQ-5D scale assessments at intervals up to 12 months post randomisation.
Ethics and dissemination This trial was approved by the Human Research Ethics Committee of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine prior to patient recruitment (approval number: 2023-076). Results from this trial will be published in peer-reviewed journals. Results will also be presented at international conferences.
Trial registration number Chinese Clinical Trial Registry (ChiCTR) Identifier: ChiCTR2300073385. Prospectively registered on 10 July 2023.
- Hip
- SURGERY
- Clinical Trial
- Protocols & guidelines
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This trial uses a rigorous randomised clinical trial design to evaluate the effectiveness of synovectomy in conjunction with the FVFG procedure for people with ONFH, ensuring a methodologically robust approach to address a specific clinical question.
Randomisation is stratified by gender and uses block sizes of 4 and 6, ensuring balanced representation and rigorous allocation concealment.
The trial follows the Standard Protocol Items: Recommendations for Interventional Trials guidelines, ensuring comprehensive and standardised reporting of the trial protocol and outcomes.
Due to the nature of the surgical intervention, blinding of the surgeons is not possible, which may introduce performance bias.
As a single-centre trial conducted in a specialised hip surgery department, the generalisability of the findings to other settings or populations may be limited.
Introduction
Osteonecrosis of the femoral head (ONFH) is a condition characterised by the death of bone cells due to impaired blood flow, leading to the collapse of the femoral head and subsequent arthritis of the hip.1 2 This disease, also known as avascular or aseptic necrosis, has been increasingly identified worldwide, affecting mainly young to middle-aged adults and significantly impacting their quality of life.3 4 In China, there were an estimated 8.12 million patients with ONFH in 2019.5 Various treatments aimed at delaying disease progression and averting total hip arthroplasty (THA) have been explored, including core decompression, non-vascularised bone grafts, and osteotomies, etc. Yet, the success of these interventions has been inconsistent, highlighting the need for more reliable solutions.
With advancements in microsurgery, the free vascularised fibula graft (FVFG) has emerged as an auspicious approach, especially in the precollapse stages of ONFH.6 7 This technique not only aims to halt the degenerative process but also seeks to restore normal hip function by leveraging the osteoinductive and osteoconductive properties of the graft.8 FVFG has demonstrated significant success in decompressing the femoral head, excising necrotic bone and augmenting revascularisation and neo-osteogenesis.7 Despite its technical demands, FVFG is increasingly recognised for its potential to improve patient outcomes by enhancing functional recovery and alleviating pain.9 10
However, existing literature and practice have primarily focused on addressing the necrotic process within the femoral head, with little consideration given to the synovial environment within the hip joint capsule. Synovitis, or inflammation of the synovial membrane, could play a crucial role in the progression of ONFH, yet its management alongside FVFG remains underexplored.11 12 The potential benefits of synovectomy, or surgical removal of inflamed synovial tissue, in conjunction with FVFG have not been systematically studied.
Objectives
Primary objective
The primary aim of this single-centre, two-arm, randomised clinical trial is to assess whether integrating synovectomy with FVFG treatment significantly improves outcomes in young adults with early-stage ONFH, specifically in terms of 12-month post-surgery Harris Hip Scores (HHS), compared with FVFG alone.
Secondary objectives
The secondary objectives of this trial are to compare the two management strategies regarding hip pain, psychological situation and quality of life at baseline (before surgery), 1, 3, 6 and 12 months after the surgery.
Methods
Trial design
This is a parallel-group, single-centre, two-arm, randomised clinical trial. Participants will be randomised and allocated into two groups: the synovectomy FVFG group and the control group receiving FVFG alone. The trial was approved by the Ethics Committee of Shanghai Sixth People’s Hospital (approval number: 2023-076). All participants provided informed consent (see online supplemental material) before entering the trial.
Supplemental material
The design of this trial follows the recommendations of the Standard Protocol Items: Recommendations for Interventional Trials guidelines (2013).13 The primary outcome will be the change in patients’ HHS 12 months after surgery. Secondary outcomes will include the affected hip’s HHS Numerical Rating Scale (NRS) for pain; Depression, Anxiety, and Stress Scale-21 Items (DASS-21) score; and EuroQol five-dimensional (EQ-5D) scale at 1, 3, 6 and 12 months post surgery.
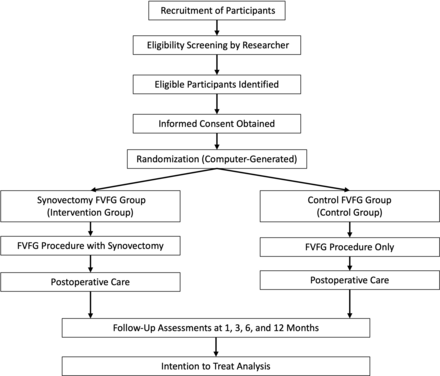
The trial was registered with the Chinese Clinical Trial Registry. The flow diagram of this trial protocol is shown in figure 1. Table 1 outlines the schedule for enrolment, screening, interventions and assessments.
Flow diagram of the study protocol.
Standard Protocol Items: Recommendations for Interventional Trials diagram of enrolment, interventions and assessments for the trial
Trial setting
Participants are recruited from the outpatient clinic of the Hip Surgery Department at Shanghai Sixth People’s Hospital, affiliated with Shanghai Jiao Tong University School of Medicine in Shanghai, China. The department is one of the pioneering centres for FVFG in China, with extensive experience in treating ONFH using this procedure. Approximately 10 000 ONFH outpatients visit the department annually, with about 300 opting for the FVFG procedure.
Public participants will be recruited through the Orthopaedic outpatient clinic at our hospital, advertisements on the hospital website, social media platforms and word of mouth. Interested individuals will contact the research team and undergo further eligibility screening by the researcher. Eligible participants will receive a participant information sheet and provide written informed consent.
No additional recruitment measures will be taken, and individuals with direct relationships to the researchers, such as students, staff of the hospital or close relatives, will not be included. Participant enrolment began in July 2023 and is scheduled to end in December 2025. These conditions establish a solid foundation for the feasibility of this trial.
Participants
This trial will recruit 90 participants in total. The trial population consists of participants with mild to moderate ONFH, and participants with significant medical illnesses are excluded to ensure safety and minimise confounding factors unrelated to the intervention. Diagnosis is based on presenting symptoms, routine clinical assessment and examination supported by radiographic evidence (X-ray and MRI) of ONFH. T1 fat-suppressed contrast-enhanced MRI of the affected hip will be applied to evaluate synovitis.14 Participants’ experiences and feedback will continue to be collated throughout the trial. Participants are eligible for the trial if they meet all inclusion criteria below:
The patient is male or female, aged between 18 and 50.
The affected side is diagnosed with ONFH according to Association Research Circulation Osseous (ARCO) stages I, II and IIIA.
The affected side has synovitis, identified by MRI.
Symptom duration is at least 6 months.
Moderate to severe hip pain (a score of 3 or more on a 0–10 numeric rating scale (NRS), where 0 indicates ‘no pain’ and 10 indicates ‘worst pain possible’).
Informed written consent was provided by the patient.
Participants will be excluded if they meet one of the criteria below:
Any previous surgery on the affected hip.
Current or previous infection of the affected hip.
Pregnant or lactating women.
Any history of mental disorder or disease.
Inability to understand and complete self-report questionnaires in Chinese.
Plan for total hip arthroplasty in the affected hip within the next 6 months.
Any history of osteoarthritis or Paget disease of the affected hip.
Presence of any haemorrhagic disease or current use of anticoagulants (eg, warfarin, dabigatran, rivaroxaban, apixaban, low molecular weight heparin), ritonavir or cobicistat.
Significant illness (have or have had) including but not limited to cancer.
Randomisation
Participants will be randomised into two groups: the synovectomy FVFG group and the control group receiving FVFG alone. Randomisation will be stratified by sex to ensure a balanced representation of men and women in each group. A computer-generated random sequence using block sizes of four and six will be used to ensure allocation concealment within each gender stratum.
An independent statistician who is not involved in the recruitment or intervention procedures will generate the allocation sequence. To maintain allocation concealment, sequentially numbered, opaque, sealed envelopes (SNOSE) containing the group assignments will be used. These envelopes will be prepared by the independent statistician and only be opened by the surgeon at the time of surgery. This method ensures that both participants and investigators remain unaware of group assignments until the intervention is administered.
Blinding
Due to the nature of the surgical intervention, it is not possible to blind the surgeons performing the procedures. However, blinding will be maintained for the participants, outcome assessors and data analysts. Participants will not be informed of their group assignment to reduce potential bias in self-reported outcomes. Outcome assessors, who will be responsible for collecting follow-up data, will be blinded to the group assignments to ensure an unbiased assessment of the primary and secondary outcomes. Data analysts will also be blinded to the group assignments during the analysis phase to prevent bias in data interpretation. By implementing these blinding measures, the trial aims to minimise bias and ensure the reliability and validity of the results.
Interventions
The trial investigates the efficiency of synovectomy as an intervention within the context of a routine and well-established surgical procedure for patients with ONFH. Participants in both the synovectomy and control groups will undergo FVFG surgery on the affected hip. The interventions will be carried out in the Hip Surgery Department (within the department of orthopaedics) at Shanghai Sixth People’s Hospital, using consumables from the same manufacturer for all procedures. The surgeries will be performed according to a standardised protocol by a senior orthopaedic surgeon with at least 5 years of FVFG experience, who performs over 50 FVFG surgeries annually. The technique used will follow previously detailed methods.15
Eligible participants will be randomised into two groups: the experimental group (undergoing synovectomy+FVFG) and the control group (undergoing FVFG only). Participants will receive written information about the procedures.
Postoperatively, all patients will receive an intravenous infusion of dextran for 3 days, followed by a daily dose of aspirin for 6 weeks. Operative drains, the Foley catheter and the epidural catheter will be removed on the second postoperative day. Physical therapy will also begin on this day. The average hospital stay is 4 days. Patients will remain non-weight-bearing on the operative side for 6 weeks, after which progressive weight-bearing will be allowed. Full weight-bearing is expected to be achieved by 6 months. Patients will be encouraged to start early active and passive motion of the toes and ankle, with particular emphasis on passive stretching of the great toe.
Participants will be advised to continue their usual medications during the trial. However, the use of analgesics for hip pain should be discontinued 3 days before follow-up visits, as notified by the investigator. All medication data, especially analgesics, will be recorded in the case report form at baseline and follow-up visits. Participants can follow the treatment guidelines for protective weight-bearing, traditional Chinese medicine and other physiotherapy measures as recommended by the Chinese Adult Femoral Head Necrosis Clinical Diagnosis and Treatment Guidelines (2020).
Outcomes
Trial outcome measures are presented in table 1.
Primary outcome
The primary endpoint is the change in HHS scores 12 months post surgery compared with the presurgical baseline.
The HHS was developed to assess hip surgery results and is intended to evaluate various hip disabilities and treatment methods in an adult population.16 The HHS measures dysfunction, with higher scores indicating better outcomes. The maximum score possible is 100. Scores can be interpreted as follows: <70=poor result; 70–80=fair, 80–90=good and 90–100=excellent.17 In this trial, HHS will be measured at baseline (before surgery), 1 month, 3 months, 6 months and 12 months after the surgery. This will allow for a comprehensive analysis of the trajectory of hip function recovery over the entire 12-month follow-up period.
Secondary outcome
Secondary endpoints include the affected hip’s HHS, NRS for pain, DASS-21 and EQ-5D score at each follow-up point (baseline (before surgery), 1 month, 3 months, 6 months and 12 months post surgery).
The NRS rates pain from 0 (no pain) to 10 (worst pain). Advantages of NRSs include simplicity, reproducibility, ease of comprehension and sensitivity to small changes in pain.18
The DASS-21 is a set of three self-report scales designed to measure the emotional states of depression, anxiety and stress.19 Each of the three scales contains seven items, divided into subscales with similar content. The depression scale assesses dysphoria, hopelessness, devaluation of life, self-deprecation, lack of interest/involvement, anhedonia and inertia. The anxiety scale assesses autonomic arousal, skeletal muscle effects, situational anxiety and subjective experience of anxious affect. The stress scale assesses chronic nonspecific arousal, including difficulty relaxing, nervous arousal and being easily upset/agitated, irritable, over-reactive and impatient. Scores for depression, anxiety and stress are calculated by summing the relevant items.
The EQ-5D is a standardised measure of health-related quality of life encompassing five dimensions: Mobility, Usual Activities, Self-care, Pain and Discomfort, and Anxiety and Depression.20 Three levels of problems are described in each dimension, representing no, moderate or extreme problems in the Pain and Discomfort and Anxiety and Depression dimensions and no, some and inability to in the Mobility, Usual Activities and Self-care dimensions.
Post-trial care
Through protective weight-bearing practices and other physiotherapy treatments, it is hoped that participants will experience physical benefits such as pain reduction. At the end of the trial, participants who have not shown improvement or have further collapse of the femoral head will undergo further examination of the hip, including MRI, if necessary, to evaluate the progression of the disease. If the patient’s hip pain continues to worsen, other surgical procedures, such as hip replacement, may be considered.
Follow-up and end of trial
Participants can withdraw from the research at any time and for any reason. If a patient chooses to withdraw from the trial before its completion, their data will still be analysed using the intention-to-treat (ITT) approach. The principal investigator will end a participant’s involvement if the participants experience a severe adverse event or do not consent to the use of the already collected data. In such cases, the data of these participants will be deleted accordingly.
Follow-ups will occur at 1, 3, 6 and 12 months postoperatively. These follow-ups will include evaluations at the outpatient department and patient-reported questionnaires. The following data will be collected and stored:
Date of intervention completion or trial withdrawal.
Reason for trial withdrawal.
Surgical information (eg, duration of surgery, blood loss).
Length of hospitalisation (in days).
Harris Hip Score after 1, 3, 6 and 12 months postoperatively.
NRS for pain at 1, 3, 6 and 12 months postoperatively.
DASS-21 at 1, 3, 6 and 12 months postoperatively.
EQ-5D score at 1, 3, 6 and 12 months postoperatively.
Analgesics use at 1, 3, 6 and 12 months postoperatively.
Adverse events at 1, 3, 6 and 12 months postoperatively.
Hip X-ray at 1, 3, 6 and 12 months postoperatively.
Trial withdrawal
Participants will be informed that they are free to withdraw from the trial at any time, including before, during and after all data collection sessions. Additionally, a participant may be withdrawn from the research for the following reasons: (1) loss to follow-up or (2) withdrawal of consent.
The reason for withdrawal will be recorded on the trial change of status form. If a participant withdraws from the trial, the data already collected will be retained and all trial outcomes will continue to be followed.
Adverse event
An adverse event (AE) is defined as any problem that occurs after a patient or clinical trial participant receives a treatment but is not necessarily related to the treatment. Severe adverse events (SAEs) are defined as events that are both unexpected and related to the trial treatments and result in hospitalisation, prolonged hospital stay, persistent or permanent harm, death, congenital abnormality or birth defect, or are life-threatening.
AEs will be collected through self-reporting by participants during the trial and by the investigator’s follow-up. The following information will be recorded for each AE: the nature of the event, the time it occurred, its duration, any actions taken and its relationship to the treatment. If laboratory tests reveal abnormal results, participants will be followed up until the test results return to normal or are determined to be unrelated to the intervention. The use of co-interventions (FVFG) will be recorded at 1, 3, 6 and 12 months.
Sample size calculation
The sample size was determined to detect a 10-point minimum clinically important difference on the HHS at the 12-month follow-up, selected for its established clinical significance in previous studies at a single assessment point.21 Using an outcome superiority design principle, with a power of 80% and a significance level (α) set at 0.05 (two-sided), the effect size reflects the clinically significant difference expected from the treatment.22 The SD of clinical change is estimated to be 5.23
To account for a dropout rate of 10%, 45 participants will be recruited for each group, resulting in a total of 90 participants.
Statistical analysis
Trial outcomes will be quantitative. The primary outcome will be the change in HHS before and after treatment (12 months after the surgery), while the NRS for pain, DASS-21 and EQ-5D scale will be considered secondary outcomes. Baseline data will include age, gender, BMI, educational level, EQ-5D score, reason for ONFH, HHS, surgery score, DASS-21 score, AEs collection and analgesics use.
The trial’s primary success will rely on comparing the change in HHS between baseline and 12 months after the surgery. In both groups, HHS at months 1, 3, 6 and 12 will be recorded and compared to explore the evolution of HHS in response to the synovectomy intervention. All statistical procedures will be conducted according to the ITT principle, with all randomised participants retaining their original randomised group. To appropriately analyse these repeated measures, we will employ linear mixed models that consider both fixed effects (treatment, time and treatment by time interaction) and random effects (subject-specific intercepts and slopes). This method will allow us to evaluate changes within individuals over time and between treatment groups, providing a comprehensive analysis of the intervention’s effectiveness at different stages of the trial. Similarly, secondary outcomes will be analysed using the same longitudinal approach, with specific models adjusted for each type of outcome measure to reflect its unique characteristics and measurement scales.
Continuous variables, including descriptive data and baseline and follow-up outcome measures, will be presented as mean values (SD), while categorical variables will be presented as counts (percentages). Quantitative data will be analysed using Student’s t-tests to compare the two groups. Data on adverse events will be analysed using the χ² test.
A preplanned analysis based on the ARCO stages will be performed to determine whether there are differences between the subgroups. Additionally, adjustments for baseline covariates may be conducted to address any imbalances between the two groups. Additionally, we will perform a subgroup analysis to examine the effects of synovectomy based on the underlying causes of synovitis, specifically stratifying participants by their history of steroid use.
No interim analyses are planned for this trial. If the proportion of missing data exceeds 5%, missing outcome data will be imputed using linear mixed modelling under the assumption of missing at random, following which the maximum likelihood estimate will be calculated. Data will be collected by the investigator at the following stages: baseline, first follow-up at month 1, secondary follow-up at month 3, third follow-up at month 6 and final follow-up at month 12.
We will include all AEs reported during the trial in our analysis to comprehensively assess the safety profile of the interventions. Each AE will be classified by an independent safety monitoring board as either related or unrelated to the intervention, based on predefined criteria. Descriptive statistics will summarise the incidence, type and severity of AEs, providing insights into the safety of the procedures for all participants.
Statistical significance will be set at the two-sided 5% level, with corresponding CIs derived. To address multiple testing and control type I error across the multiple outcomes in this trial, we will adjust the significance thresholds using the Bonferroni correction to maintain the overall type I error rate at 0.05. For exploratory and subgroup analyses, the Benjamini-Hochberg procedure will be used to control the false discovery rate, allowing for a balanced approach to identify significant findings without being overly conservative. R software (V.4.4.0) will be used for all statistical analyses performed by statisticians blinded to the clinical trial.
Data management and confidentiality
The trial results will be published in a medical journal. Each participant will be assigned a unique project number to ensure anonymity. Data will be collected using a case report form (CRF), which will include basic participant information. In a significant improvement to enhance data integrity and security, all electronic records will be managed through an Electronic Data Capture (EDC) system provided by our hospital. This system ensures encrypted data entry and storage and allows for comprehensive audit trails which are essential for maintaining the quality and veracity of the trial data. A Data Monitoring Committee (DMC) will oversee the SYNERGY-VGF trial to ensure participant safety, data integrity and trial quality. The DMC will include independent experts in orthopaedic surgery, biostatistics and ethics, ensuring no conflicts of interest. The DMC operates independently from the sponsor and investigators, with members free from conflicts of interest, and they will meet biannually or as needed, reporting findings and recommendations to the trial’s leadership.
All data recorded will be securely stored in the Department of Hip Surgery at Shanghai Sixth People’s Hospital, part of the Shanghai Jiao Tong University School of Medicine in Shanghai, China, where data protection measures will be enforced. All data entries will be manually reviewed to avoid errors.
All researchers involved in the trial will sign a confidentiality agreement to protect the privacy of the participants. Participants’ medical records and CRFs will be kept in a locked cabinet for 5 years to ensure the clinical trial’s integrity and to allow for the quality of the research data to be evaluated and verified at Shanghai Sixth People’s Hospital. Personal information will not be identifiable in any published papers. Only statisticians from Shanghai Sixth People’s Hospital who are involved in this research will have authorisation to access the stored data.
Ethics and dissemination
This trial was approved by the Human Research Ethics Committee of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine prior to patient recruitment (approval number: 2023-076). The trial was registered with the Chinese Clinical Trial Registry (ChiCTR2300073385) on 10 July 2023.
The trial protocol, written informed consent forms and information directly related to the participants must be submitted to the Ethics Committee. Written approval from the Ethics Committee must be obtained before the trial can commence. The investigator is required to submit an annual report on the trial to the Ethics Committee at least once a year, if applicable. The investigator must also notify the Ethics Committee in writing when the trial is discontinued and/or completed.
Any changes to the trial protocol, such as revisions or updates to the informed consent forms, must be reported to the Ethics Committee in a timely manner. These changes cannot be implemented without the prior approval of the Ethics Committee, unless they are made to eliminate an immediate risk to the participants. If such changes are made, the Ethics Committee will be notified promptly.
Patient and public involvement
None.
Discussion
To our knowledge, this study is the first randomised clinical trial to explore the effectiveness of synovectomy during the FVFG procedure for patients with ONFH compared with a control group. Patients with ONFH are at high risk for femoral collapse, which significantly impacts their quality of life. This research aims to enhance our understanding of effective treatment options for this vulnerable patient population.
Our protocol features a robust randomised controlled design that enhances the reliability of our findings. Conducting this trial at one centre enabled stringent control of surgical procedures and patient care, which is crucial for assessing the efficacy of synovectomy with FVFG in treating ONFH. While the single-centre design ensured high-quality data collection and consistent intervention application, it limits the generalisability of the findings. Future studies should expand to multicentre trials to validate our results across diverse clinical settings, enhancing the external validity and broader applicability of this treatment approach for ONFH. We chose SNOSE for randomisation, but not an EDC system, which may limit our ability to mitigate potential biases associated with this method fully.
The results of this study will provide guidance for clinical implementation and advance the development of FVFG for ONFH. Several key issues have been addressed in the trial design to ensure robust and reliable results. If synovectomy is proven to be effective, it could be considered a valuable addition to the surgical treatment regimen for ONFH, offering a safe, cost-effective and less technically demanding option. This could significantly benefit patients and improve surgical outcomes for ONFH.
Trial status
The current protocol is V.1, dated 6 May 2023. Recruitment began in July 2023 and is ongoing at the time of manuscript submission. It is expected to complete all research processes by the end of May 2025.
Ethics statements
Patient consent for publication
References
Footnotes
KF and WZ are joint first authors.
Contributors KF has written the first draft of the manuscript. WZ, QC, HP, HH, DZ, CJ, ZW, PD, JY, DJ, C-QZ, and YG were all involved in the design of the study, revision and final approval of the manuscript. YG is the guarantor of this study.
Funding This trial was funded by the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant (2022). The funder has no role in the trial design, collection, management, or writing the report.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.