Article Text
Abstract
Objectives To compare costs, health outcomes and cost-effectiveness of using intravenous lidocaine (bolus given at induction of anaesthesia, followed by infusion for 6–12 hours) during colorectal surgery to improve the return of gastrointestinal function.
Design Within-trial planned analysis of data from a randomised controlled trial using an intention-to-treat approach.
Setting 27 hospitals from across the UK.
Participants 557 patients aged 25–91 having minimally invasive elective colorectal resection.
Intervention A 1:1 randomisation between intravenous lidocaine and placebo, minimised for age (<50 years, 50–74 years, ≥75 years), gender, and trial centre.
Primary outcome measures Mean differences between trial arms in 30-day and 90-day quality-adjusted life-years (QALYs) and 30-day total National Health Service costs, as well as the 30-day incremental cost-effectiveness ratio.
Results Compliance and data quality were high. Intravenous lidocaine is associated with differences of £38 (95% CI: −£463, £589) in total 30-day costs, −0.0005 (95% CI: −0.0027, 0.0015) in 30-day QALYs and −0.0008 (95% CI: −0.0066, 0.0048) in 90-day QALYs. No large, statistically significant or meaningful differences in primary or secondary outcome measures between trial arms were detected, other than for the intervention costs.
Conclusion Intravenous lidocaine is not found to impact costs or health outcomes for patients undergoing colorectal surgery. In the absence of a clinical effect, disinvestment from perioperative lidocaine could save costs associated with infusion monitoring.
Trial registration number International Standard Randomised Controlled Trial Number 52352431.
- Economics
- Endoscopy
- Clinical Trial
- Colorectal surgery
Data availability statement
Patient data have been managed to safeguard the confidentiality of patients, consistent with the terms of consent signed by patients. For data requests pertinent to the main clinical analysis, see online supplement 4 of the ALLEGRO clinical paper.5 All data requests specific to the health economic analysis should be submitted by the Edinburgh Clinical Trials Unit (ECTU, email: ECTUdatashare@ed.ac.uk) for consideration. Access to anonymised data may be granted following review. Any data sharing approved would have to ensure patient confidentiality.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
High-quality multicentre data collection, large sample size and low missingness.
Pragmatic trial design embedded in daily usual practice in sites throughout the UK.
Parameter uncertainty due to difficulties in sourcing accurate costs of recovery room time.
Reporting designed for use in future health economic modelling.
Introduction
Colorectal surgery is common in UK hospitals. After a segment of the colon is removed and the bowel rejoined, it can take a few days for bowel function (eating and passing flatus and stool) to recover. Almost all patients stay in the hospital until bowel function recovers. In a significant proportion of patients, bowel recovery takes longer than a few days, causing vomiting, abdominal pain and swelling.1 These patients are unable to eat until the bowel recovers, requiring supportive treatment (intravenous fluids, etc) and a longer hospital stay. There is no specific treatment to help the bowel recover faster—patients simply have to wait. Modern minimally invasive surgical techniques mean that other aspects of recovery (eg, pain control, resumption of independent mobility) are established within 48 hours. Therefore, interventions able to reduce bowel recovery time have the potential for improved patient comfort, and National Health Service (NHS) cost reduction if earlier discharge can be achieved.
Two small, single-centre randomised controlled trials (RCTs) in elective laparoscopic colonic surgery found that perioperative intravenous lidocaine accelerated bowel function recovery and reduced the length of hospital stay.2 3 Lidocaine is cheap, familiar to healthcare workers, widely used and has a well-documented safety profile. Hence, if definitively demonstrated to improve recovery time, it would have a high potential for cost-efficiency.
The ALLEGRO trial tested whether intravenous lidocaine improves recovery of bowel function after minimally invasive elective colorectal resection surgery.4 5 The trial found that perioperative administration of 2% intravenous lidocaine infusion did not improve the return of gut function at 72 hours among adults undergoing elective minimally invasive colon resection.5
Here, we present the results of the prespecified within-trial economic evaluation for ALLEGRO. The objectives of this analysis were to assess the cost-effectiveness of using intravenous lidocaine, measured in cost per incremental quality-adjusted life-year (QALY), relative to standard care as observed over the 30-day trial period from an NHS and Personal Social Services (PSS) perspective. The data quality also enables us to describe the observed patterns of healthcare utilisation and health utility during the post-surgery recovery.
Methods
Trial overview
Full details of the ALLEGRO trial, its procedures (including inclusion/exclusion criteria) and clinical findings can be found in the study protocol and main results paper.5 The following summary is included for context only.
ALLEGRO was a multicentre, pragmatic, placebo-controlled, randomised trial. Enrolment occurred from 13 August 2018 to 11 April 2023, with a pause in recruitment from 20 March 2020 through 6 July 2020 due to the SARS-CoV-19 pandemic. The final follow-up was on 10 August 2023. 557 participants from 27 UK hospitals (see online supplementay table 1) undergoing elective colonic resection for colorectal cancer, benign polyps, benign stricture or diverticular disease were randomised on a 1:1 ratio to either5 6
Supplemental material
Intravenous lidocaine: sterile solution of lidocaine 2% made isotonic with sodium chloride, or
Placebo: 0.9% sterile sodium chloride solution for injection.
An intravenous bolus of 2% lidocaine (or placebo) was administered at induction of anaesthesia over 20 min, followed by intravenous infusion for a minimum of 6 hours up to a maximum of 12 hours. The duration of the infusion was determined preoperatively by the participating units’ normal postoperative availability of continuous cardiac monitoring (mandated during the study as lidocaine toxicity manifests as cardiac arrhythmia). Exact dosing regimens are described in the ALLEGRO trial protocol.4
Economic principles
The methods of calculating costs, health outcomes and cost-effectiveness metrics were outlined in a Health Economic Analysis Plan (HEAP), including preselected price weights, signed off by the lead economist and chief investigator before data lock and unblinding. Our paper follows Consolidated Health Economic Evaluation Reporting Standards guidance for health economic evaluations as summarised in table 1.7
Consolidated Health Economic Evaluation Reporting Standards (2022) checklist
To maximise UK policy relevance, the analysis followed National Institute for Health and Care Excellence (NICE) reference specifications.8 This included the use of QALYs as the primary health outcome and the adoption of an NHS and PSS perspective with primary cost-effectiveness outcomes presented in cost-utility format in terms of incremental cost-per-QALY.8
The time horizon for measuring QALYs and costs and for producing cost-effectiveness results was 30 days. This was the last time point in which both healthcare resource utilisation (HRU) data and patient-reported outcome questionnaires were collected.4 An additional 90-day QALY was calculated beyond the 30-day time horizon but was not used in the cost-effectiveness analysis due to the absence of corresponding cost data. As all time horizons were under 1 year, no discounting was necessary for costs or outcomes.
The base year for all analyses was the financial year ending 2022, selected as the latest year for which key price weight sources were available at the time of finalising the HEAP. All analyses were undertaken on an intention-to-treat basis.
Data collection
Data collection time points were measured in postoperative days (PODs), which included the first 7 days, POD 30 and POD 90 in addition to baseline data (see online supplementay table 2 for a detailed data collection timeline). Primary and secondary care data, along with quality-of-life data, were collected from study questionnaires. Quality-of-life data collection used the validated EQ-5D-5L instrument by EuroQoL, a generic questionnaire comprised of five dimensions: ‘mobility’, ‘self-care’, ‘usual activities’, ‘pain/discomfort’ and ‘anxiety/depression’, each ranked on a Likert scale from 1 (no problems) to 5 (extreme problems).9 Other HRU data, consisting of surgery details, adverse events (AEs), complications and length of stay (LOS) were collected from Case Report Forms.
Estimating outcomes
QALYs were calculated from EQ-5D-5L instrument data.9 Data from patient self-reported EQ-5D-5L questionnaires (issued at POD: 1–7, 30 and 90) were converted into health utility scores using a mapping function recommended by NICE. This is a prescored algorithm where each combination of the five domains is allocated a health utility represented by a numerical value, where 0 is equivalent to death and 1 represents full health.8 10 11 For each patient, 30- and 90-day QALYs were calculated as a function of health utility scores and PODs using the validated area-under-the-curve formula (using PODs 1, 7, 30 and 90).12 Health utility scores from PODs 2–6 were not included in QALY estimates as they were not collected for discharged patients. These were collected opportunistically for context purposes only.
Estimating costs
Reported healthcare utilisation for each patient was combined with the corresponding price weights (shown in table 2) to estimate cost. Total costs included all costs incurred between the operation and the postoperative questionnaire of day 30.
Unit costs and price weights
The hourly cost of operating theatre use published by Public Health Scotland was identified at the review stage after the HEAP was written to replace a previous less robust source.
No specific price weight was located for recovery room time. The cost of the high-dependency unit was used as an approximation following clinical consultation. These are stratified by the number of organs supported (between 0 and 6+) in the National Cost Collection13 and 0 organs supported by reasonable approximation. This differs from the cost of intensive care time for which two organs were supported, chosen as a reasonable midway point also guided by clinical advice.
The costs of administering the placebo were excluded as they are not part of the current standard of care.
All unit costs not originally reported in 2022 GBP were converted into the 2022 prices using the Office for National Statistics’ health-specific Consumer Price Index.14
Analysis
Statistical analysis was performed using the R statistical programming language.15 For each outcome and cost variable and total costs, the unadjusted arithmetic mean and SD were reported separately for each trial arm, along with the difference in means between arms.
To account for missing data and the non-normal and skewed distribution of estimates, statistical tools from the validated bootImpute R programming package were used to estimate cost and QALY 95% CIs for means and differences in means between trial arms.16 These combined multiple imputation by chained equations, non-parametric bootstrapping and generalised linear model regression with a gamma distribution and a log link recommended by Manning and Mullahy.17 18 For both costs and QALYs, both unadjusted and adjusted results were presented; unadjusted (univariate) results contain the trial arm as the only independent variable, whereas adjusted (multivariate) regression formulas control for age, sex and intravenous lidocaine infusion duration (6 vs 12 hours).
The analysis also included exploratory post hoc observational regression outputs grouping patients by infusion duration (6 vs 12 hours) rather than the trial arm in both adjusted and unadjusted regression formulas.
As per NICE guidelines, the primary cost-effectiveness metric was incremental cost-effectiveness ratios (ICERs) in terms of incremental cost per QALY gained (intervention minus control). These were reported alongside measures of parameter uncertainty in the form of cost-effectiveness plane (CEP) scatterplots and cost-effectiveness acceptability curves (CEACs).8 The ICERs and plots were reported separately for unadjusted and adjusted results. The CEPs and CEACs were constructed by calculating incremental costs and QALYs (adjusted and unadjusted) separately for 1000 unpooled simulated data set iterations in the bootImpute function, composed of 500 bootstrap samples (nBoot=500) each imputed twice (nImp=2), as recommended by Hippel and Bartlett.16 CEP plots incremental costs against incremental QALYs, whereas the CEAC plots the probability of intravenous lidocaine being cost-effective at various willingness-to-pay (WTP) thresholds by calculating the incremental net monetary benefit for each simulated data set.
Lidocaine toxicity risk modelling
We initially planned to undertake simplified modelling of longer-term outcomes associated with intravenous lidocaine toxicity (ie, overdose due to incorrect infusion quantity administered by an anaesthetist) such as fatal cardiac arrhythmias. This was not undertaken as the matter was rendered moot by the absence of serious AE (SAE) differences and a clinical benefit to be traded off against.
Results
Study population
Out of 561 participants, 4 participants did not have an operation due to withdrawing before the day of surgery. Only participants who had an operation were included in the health economic analysis. Following the principles of an intention-to-treat analysis, patients who did undergo an operation but did not receive intravenous lidocaine are included in the study. The study population included in the economic evaluation analysis is presented in table 3. Patients were recruited from 27 centres across the UK (see online supplementary table 1).
Patient population
Data quality
The ALLEGRO study is overall characterised by a low degree of missing data (see online supplementary table 3). For health resource use (and subsequently cost categories), the missingness did not exceed 5% of patients, while missingness was below 20% for health utility scores at baseline and PODs 1, 7, 30 and 90. The degree of missingness is higher by design for health utility score data collected between POD 2 and 6 as patients who were discharged were not issued surveys.
12 participants in the intravenous lidocaine arm did not receive lidocaine. This was due to reasons such as investigational medicinal products (IMP) logistics (where the drug was not available to give to the participant) and anaesthetist preference (where the anaesthetist did not want to proceed with the infusion).
Table 4 presents the results of the primary health economic outcomes measured in the ALLEGRO trial, including health utilities, QALYs and costs. A breakdown of HRU rates from which costs were calculated is provided in online supplementary table 4. Health utility score progression over time is visualised in figure 1. Table 4 results are univariate, except for the key results of 30-day QALYs and total costs, for which regression-adjusted (multivariate) estimates are provided. All costs in table 4 were measured within a 30-day time horizon.
Results (means, mean differences, 95% CIs and p values)
Mean health utility score progression by pos-operative day. IV, intravenous
There was no statistically significant difference in most health utility scores and all cumulative QALYs between trial arms at the 95% confidence level at any time point. The only exception is a small but statistically significant difference of −0.0481 (–0.0907, –0.0044) in the health utility score on POD 3, which had subsided by POD 7. This was not enough to influence the QALY results and may be an artefact of not adjusting for repeated measures.
Differences between trial arms in mean per-patient total costs were not statistically significant at the 95% confidence level. For individual HRU categories, only three items had statistically significant differences:
Intravenous lidocaine: patients in the intravenous lidocaine consumed £18 worth of intravenous lidocaine which was not used in the placebo arm by definition.
General practitioner (GP) surgery consultations: the mean (95% CI) difference in GP surgery consultations is −£5 (−£9, £0) in the intravenous lidocaine arm compared with the placebo arm.
Unplanned admissions: the mean (95% CI) difference in unplanned admission costs is −£8 (−£12, −£1) in the intravenous lidocaine arm compared with the placebo arm.
It should be noted that the detected differences in GP surgery consultation and unplanned admission costs, while technically statistically significant, were negligible. These results should not be subject to overinterpretation as they have not been adjusted for repeated measures and are likely to be artefacts of the heavily skewed nature of cost distributions.
None of the differences in adjusted results presented in table 4 are statistically significant at the 95% confidence level.
Effect of infusion duration
The post hoc regression analysis shows a statistically significant difference in QALYs at the 95% confidence level, with a decrease in QALYs with shorter infusion times, and a large statistically significant reduction in costs for 6-hour infusion times (see online supplementary table 5). The difference in cost is most likely due to the shorter infusion, by definition, as well as the shorter time in recovery. The QALY difference between 12-hour and 6-hour centres may be confounded by a difference in patient profiles between centres or different perioperative care procedures between centres. These analyses are non-randomised and are intended to be speculative and hypothesis-forming only.
Cost-effectiveness
The main measure of cost-effectiveness is the ICER. However, in both unadjusted and adjusted results, costs were higher and QALYs lower in the intravenous lidocaine arm; so, the ICERs could not be calculated as intravenous lidocaine is dominated by standard care as a treatment strategy.
Uncertainty around cost-effectiveness estimates was measured using non-parametric bootstrapping and visualised in CEPs in figure 2 for unadjusted and adjusted estimates. Each point on the CEP represents the incremental costs and QALYs (intravenous lidocaine—placebo) of a given simulated bootstrap data set. In both unadjusted and adjusted CEP plots, results span all four quadrants and the base case point is close to the origin. This is expected given the lack of statistically significant differences in mean QALYs and total costs between trial arms.
Cost-effectiveness plane. QALY, quality-adjusted life-year; WTP, willingness-to-pay.
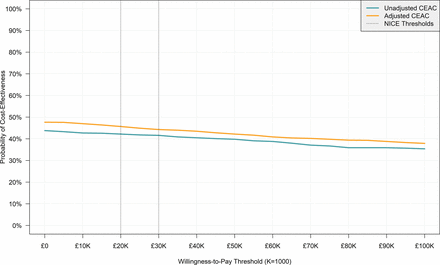
The percentage of simulated observations in each CEP quadrant for both adjusted and unadjusted estimates and the percentage of simulated observations under a £20 000 and £30 000 WTP threshold are presented in table 5. Figure 3 presents the CEACs for unadjusted and adjusted results. In both cases, the probability of intravenous lidocaine being cost-effective decreases with the WTP. Nevertheless, the probability is below 50% for both adjusted and unadjusted estimates throughout the WTP, as is expected given the lack of statistically significant differences in mean QALYs and total costs between trial arms.
Cost-effectiveness results
Cost-effectiveness acceptability curve (CEAC). NICE, National Institute for Health and Care Excellence.
Discussion
Summary
The results of the ALLEGRO health economic analysis are consistent with the clinical results showing no effect of intravenous lidocaine. The differences in total costs and QALYs between placebo and intravenous lidocaine are small and not statistically significant. This was unaffected by a small but statistically significant difference in the health utility score on POD 3 (see table 4), which itself is likely an artefact of not adjusting for repeated measures. Visualisation of uncertainty of the CEP plots shows a spread of simulated cost-effectiveness results across the CEP quadrants, confirming that there is likely no difference between trial arms other than random chance.
Interpretation of the results should account for the relative importance of key price weights in determining total costs and cost differences. The low cost of the IMP (~£18 per patient) rendered its contribution to total costs overwhelmed by the noise of a wide range of non-significant but higher-cost HRU factors (eg, unscheduled assessments).
Our post hoc analysis did, however, find a statistically significant improvement in both costs and QALYs of centres with a 6-hour infusion duration (of either IMP or placebo) over those of 12 hours, irrespective of the trial arm. However, since the results of this study point to lidocaine having no benefit over standard care, the duration of the infusion is therefore rendered irrelevant. Furthermore, we advise caution in the interpretation of these results as they were not randomised and do not account for differences in patient populations or surgeon experience between sites. We include these as potentially hypothesis-forming outputs for future research considering other infusions only.
The decision not to proceed with building the model was based on the following two considerations: (1) simulating the effects of low-frequency and high-impact events was deemed unnecessary given a lack of difference in SAE rates between trial arms, and (2) simulating long-term costs and outcomes of using intravenous lidocaine following colorectal surgery was deemed unnecessary given no statistically significant difference in costs and outcomes between arms within the trial time horizon.
Strengths and limitations
The strengths of the ALLEGRO economic evaluation lie in the detailed data collection, both for quality of life and cost outcomes, with relatively low missingness (see online supplementary table 3). HRU variables (and thus costs) are characterised by low missingness (<10%) for both arms. The EQ-5D-5L-derived health utility score is a composite measure that depends on multiple elements of the patient questionnaire and thus has higher missingness. Health utility score missingness is higher for PODs 2–6 (as they were only completed in-hospital so people who had been discharged did not have the opportunity to complete them), which are excluded from QALY calculations, and lower (<20%) for PODs 1, 7, 30 and 90. Data quality further benefits from the size of the trial, the broad range of hospital contexts included and high compliance.5
A further advantage of ALLEGRO is that it is a relatively unique study as there is limited health economic literature related to lidocaine for colorectal surgery recovery. A rapid (non-systematic) PubMed literature search was conducted to search relevant literature on the use of lidocaine or alvimopan (a drug found in RCTs to improve bowel recovery) in colorectal surgery, which identified two protocols for lidocaine RCTs (one of which is ALLEGRO) but no complete RCTs, and four observational studies related to alvimopan.4 19–24 In contrast to the results of lidocaine’s effect in this study, alvimopan is reported to reduce LOS and costs following colorectal surgery.22
The health economic analysis of ALLEGRO shares the limitations of its main clinical analysis, which include a lack of information about participant race, ethnicity or socio-economic status, and the exclusion of more complex colorectal operations (eg, low rectal cancer).5
The cost results are further subject to a degree of parameter uncertainty (see table 2). For example, the hourly cost of the recovery room was estimated by proxy, but the difference in costs was small and statistically insignificant. In this case, more accurate price weights are unlikely to meaningfully change the results of our analysis. While we acknowledge this limitation of the study, future research in this area should consider micro-costing the recovery room time and procedure to produce more accurate cost parameters and results.
Conclusion
While both the clinical trial and the health economic analysis show no effect of intravenous lidocaine, this article is an important contribution to the literature due to the robustness of the trial warranting more definitive statements that lidocaine does not affect gut recovery in this patient population. We hope our results will counteract publication bias that may help guide future research funding. Similarly, with low missingness, the publication of HRU results and reported health utility scores in this article can be a potentially very useful source of parameter estimates to aid future modelling of postoperative colorectal surgery, and we have included details within our results tables to aid such reuse.
We present robust data strongly indicating intravenous lidocaine is not found to impact costs or health outcomes for patients undergoing colorectal surgery, other than the lidocaine infusion itself. In the absence of clinical effects, disinvestment from perioperative lidocaine could save costs associated with infusion monitoring. Future research may wish to focus on alternative strategies for the return of gut function and duration of recovery time.
Data availability statement
Patient data have been managed to safeguard the confidentiality of patients, consistent with the terms of consent signed by patients. For data requests pertinent to the main clinical analysis, see online supplement 4 of the ALLEGRO clinical paper.5 All data requests specific to the health economic analysis should be submitted by the Edinburgh Clinical Trials Unit (ECTU, email: ECTUdatashare@ed.ac.uk) for consideration. Access to anonymised data may be granted following review. Any data sharing approved would have to ensure patient confidentiality.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by West of Scotland 1 Research Ethics Committee (17/WS/0210) and the MHRA (CT 01384/0255/001). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The completion of the ALLEGRO health economic evaluation would not have been possible without the dedication of staff at each of the 27 centres which supported the trial. We are also grateful to the patients who participated in this trial and generously agreed to share time and trust. We would also like to thank the study sponsors for the funding and support needed to conduct this research. This work has collaboratively advanced medical research, and we would like to thank everyone involved in its support.
Footnotes
Contributors MA (research fellow) and TV (research fellow) contributed to the management activities to annotate (produce metadata), scrub data and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later re-use; application of statistical, mathematical, computational and other formal techniques to analyse or synthesise study data; development and design of methodology; creation of models; preparation, creation and presentation of the published work, specifically visualisation/data presentation; and preparation, creation and presentation of the published work, specifically writing the initial draft, critical review, commentary and revision. AS (senior health economist) acted as the guarantor and contributed to the formulation of ideas and evolution of overarching research goals and aims; management activities to annotate (produce metadata), scrub data and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later reuse; application of statistical, mathematical, computational and other formal techniques to analyse or synthesise study data; acquisition of the financial support for the project leading to this publication; development and design of methodology; creation of models; preparation, creation and presentation of the published work, specifically visualisation/data presentation; and preparation, creation and presentation of the published work, specifically writing the initial draft, critical review, commentary and revision. SC (senior trial manager) and KI (trial manager) contributed to conducting a research and investigation process, specifically data/evidence collection; development and design of methodology; creation of models; management and coordination responsibility for the research activity planning and execution; oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team; and preparation, creation and presentation of the published work, specifically writing the initial draft, critical review, commentary and revision. AB (team lead—ERAS, Stoma and Prehabilitation services) contributed to conducting a research and investigation process, specifically data/evidence collection; development or design of methodology; creation of models; management and coordination responsibility for the research activity planning and execution; oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team; and preparation, creation and presentation of the published work by those from the original research group, specifically critical review, commentary and revision. RA (PPI member), IF (consultant anaesthetist), SN (consultant anaesthetist, retired) and DS (consultant colorectal surgeon) contributed to the formulation of ideas and evolution of overarching research goals and aims; acquisition of the financial support for the project leading to this publication; and preparation, creation and presentation of the published work by those from the original research group, specifically critical review, commentary and revision. LA (senior research fellow) contributed to the development or design of methodology; creation of models; and preparation, creation and presentation of the published work by those from the original research group, specifically critical review, commentary and revision. ZB (data coordinator) contributed to conducting a research and investigation process, specifically data/evidence collection; development and design of methodology; creation of models; management and coordination responsibility for the research activity planning and execution; and preparation, creation and presentation of the published work, specifically writing the initial draft, critical review, commentary and revision. GML (professor/CHaRT director) contributed to the application of statistical, mathematical, computational and other formal techniques to analyse or synthesise study data; development or design of methodology; creation of models; oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team; and preparation, creation and presentation of the published work by those from the original research group, specifically critical review, commentary and revision. JN (professor/ECTU director) contributed to the formulation of ideas and evolution of overarching research goals and aims; application of statistical, mathematical, computational, or other formal techniques to analyse or synthesise study data; acquisition of the financial support for the project leading to this publication; and preparation, creation and presentation of the published work by those from the original research group, specifically critical review, commentary and revision. HP (consultant colorectal surgeon) contributed to the formulation of ideas and evolution of overarching research goals and aims; acquisition of the financial support for the project leading to this publication; conducting a research and investigation process, specifically data/evidence collection; development and design of methodology and the creation of models; management and coordination responsibility for the research activity planning and execution; oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team; and preparation, creation and presentation of the published work, specifically writing the initial draft, critical review, commentary and revision.
Funding This study/project is funded by the NIHR Health Technology Assessment Programme (NIHR 15/130/95). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests AB declares personal payment from Encare as an ERAS Implementation Coach. AS, GML, JN, LA and SC report grant funding to institution from NIHR. JN also reports the following roles: chair of MRC/NIHR Efficacy and Mechanism Evaluation Board 2019–present; HTA Commissioning Sub-Board (EOI) 2016–2017; NIHR CTU Standing Advisory Committee 2018–2023; NIHR HTA and EME Editorial Board 2015–2019; Pre-Exposure Prophylaxis Impact Review Panel 2017; EME—Funding Committee Members 2019–2022; EME Strategy Advisory Committee 2019–present; HTA General Committee 2016–2019; HTA Post-Funding Committee teleconference 2016–2019 and HTA Funding Committee Policy Group 2016–2019. IF reports payment or honoraria for a lecture from MSD. Other authors have no disclosures of interest to report.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of the ALLEGRO clinical trial. No additional patient and public involvement was undertaken specifically for the trial’s health economic evaluation, beyond that of the main trial (see our clinical paper for details). Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.