Article Text
Abstract
Objectives COVID-19, a public health emergency affecting the world in 2019, not only greatly promoted the development and application of vaccines but also effectively shortened the publishing time of scientific papers. In view of these facts, the current situation, status, problems and development trends of vaccine research and application were explored through bibliometric analysis of highly cited papers in the vaccine field within the time frame of 2014–2024, and the countries, institutions, authors, funding agencies and other relevant information that contributed most to vaccine research and application were summarised.
Design Bibliometric analysis through data analysis and visual mapping.
Data sources Scientific articles.
Data extraction and synthesis ‘Vaccine’ and ‘vaccines’ were used in the WoS database to retrieve the publications and to adequately collect the data; Microsoft Excel was used for data analysis; and VOSviewer was used for visual description of data. Overall publication trends, countries, institutions and funding agencies, authors and articles, journals and languages, and research areas and co-occurrence keywords were analysed by bibliometrics.
Results A total of 3258 highly cited papers were published in the field of vaccines in the past decade, from 735 different journals. With the COVID-19 pandemic in 2019, the number of highly cited papers in the field of vaccine research increased significantly from 2020 to 2024, accounting for 76.12%. The number of highly cited papers for vaccines peaked in 2021 and 2022, followed by a rapid decline. Highly cited papers came from 7133 institutions in 153 countries, and the most influential country in the field of vaccines was the USA, which published 1733 highly cited papers, accounting for 53.19% of the highly cited papers. The top 15 institutions with the largest influence were all from the USA or UK with 2567 published papers in total, accounting for 78.79% of highly cited papers. 4787 funding agencies were recognised in funding 2368 highly cited papers. A total of 30 926 authors in 90 research areas contributed significantly to global vaccine research. The most highly cited paper was ‘Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine’ from the New England Journal of Medicine, which was cited 9435 times in total. Among the 9848 co-occurrence keywords, COVID-19 (including SARS-CoV-2, 2019-COVID and SARS2) was the most frequently co-occurrence keyword. It appeared in 1720 articles, accounting for 52.79%, indicating that COVID-19 was the most popular study in the last decade.
Conclusions This study visualised the research and application of vaccines in the world from the perspective of papers output, drew the knowledge map and identified the important research hotspots and development trends in the vaccine field in the recent 10 years (2014–2024), which is helpful for Centres for Disease Control and Prevention, clinicians, researchers and health policymakers to better understand the research status and problems in vaccine research and application and predict its future development direction.
- COVID-19
- Public health
- BIOTECHNOLOGY & BIOINFORMATICS
- Vaccination
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This innovative study examines the development of all types of vaccines globally and explores the development of the entire vaccine field.
We used a variety of methods (including visual analysis techniques and mathematical prediction methods) and analysed the literature in the field of vaccines from multiple perspectives (eg, ‘Publication trends’, ‘Countries, Institutions and Funding Agencies’, ‘Authors and Researches’, ‘Journals and Languages’ and ‘Research Areas and co-occurrence Keywords’).
By analysing the highly cited papers, it is possible to better grasp the research hotspots in the field of vaccines.
As we only collected relevant publications from the WoS database, this may have led to an underestimation of the volume of paper outputs.
Some paramount papers may have not yet accumulated enough citations due to their recent publication, which may result in less comprehensive statistics for highly cited papers.
Introduction
British doctor Edward Jenner invented the smallpox vaccine in 1796. He took the smallpox virus (cowpox) from cattle and inoculated people with it to make them immune to smallpox. In 1885, French scientist Louis Pasteur invented the rabies vaccine. He found that the rabies virus could be cultured in the laboratory for a long time, so he weakened the virus and made a rabies vaccine that could stimulate the human body to produce immunity. The invention of vaccines peaked in the early 20th century. Yellow fever vaccine, plague vaccine, poliomyelitis vaccine and whooping cough vaccine, among others were invented successively.1 2 At this point, vaccination has become one of the most successful public health interventions and a cornerstone of infectious disease prevention.3 4
The arrival of COVID-19 in 2019 has greatly stimulated the global vaccine research and development boom.5 The time from research and development to application of the COVID-19 vaccine was greatly shortened, but the research on the protective effect and safety of the vaccine and the suitable population of vaccine inoculation is obviously lagging behind. The research on vaccine research and application from the perspective of paper output is also relatively insufficient. In recent years, a large number of predatory journals have emerged,6 and the number of publications related to vaccine research has further increased, but it does not mean that the research quality has improved or the research content has deepened.7 We found that 32 064 papers related to vaccine research in the recent 10 years were not cited by any papers, accounting for 17.39%, which not only caused a great waste of scientific research funds but also made it challenging to predict the trend of vaccine research and comprehensively evaluate its quality.8 9 Therefore, based on the analysis of Essential Science Indicator (ESI) highly cited papers, we attempted to explore the research hotspots and trend prediction of global vaccine research and application from 2014 to 2024 through multi-dimensional cluster analysis of ESI highly cited papers. What is an ESI highly cited paper? Highly cited papers refer to papers that rank in the top 1% of citations within the same year and subject, based on citation data from the past 10 years.10 Because highly cited papers are updated every 2 months, they can not only represent the major progress of vaccine research in terms of quality but also enable researchers to timely track the international trend of vaccine research. Here, we use the bibliometric analysis method for the research of paper output. The bibliometric analysis focuses on the measurement and quantification of various characteristics of publications by retrieving the theoretical research results of researchers, affiliated institutions and countries in a specific field, and using mathematical and statistical methods for data analysis or knowledge mapping.11 12 Bibliometrics research has an important value, it can not only determine the core literature of a certain field but also evaluate the publication, investigate the utilisation rate of literature and realise the scientific management of library and information department. In a larger scope, it also helps design a more economical information system and network, improve the efficiency of information processing, find the drawbacks and defects in literature service, predict the direction of publication, develop and improve the basic theory of information, describe the future research hotspots and frontier areas of scientific research and provide a comprehensive picture of the development status of this field for policymakers, clinicians and researchers. Therefore, bibliometric analysis may be suitable for exploring the characteristics of highly cited studies in vaccine research. Comparing the trends in the affiliation of different countries and institutions in the output of papers may help formulate science policy and strengthen research management.13
Existing studies are inadequate since there are few bibliometrics studies to introduce the current situation of vaccine research and application at home and abroad. What is more common is the visual analysis of specific vaccine research and application, including COVID-19 vaccines, RNA vaccines, parasite vaccines, SARS-CoV-2 vaccine, ebola vaccine, virus vaccine, HIV vaccine, anthrax vaccine or cancer vaccines.14 Global vaccine research overview and overall situation analysis based on ESI highly cited papers are rare. Through bibliometric analysis of highly cited papers on global vaccine research and application within the time period 2014–2024, this study aims to find out the trend and status of vaccine research; excavate relevant information of countries, institutions, authors and funding institutions that contribute the most to vaccine research; and get a glimpse of the output trend and rule of vaccine research papers to provide reference for vaccine research and design, as well as ideas for the collection of vaccine research literature sources, contribution for researchers and construction for vaccine research-related journals.15 Through the above analysis, this study may be able to forecast the development of the vaccine field and also serve as a guideline to help researchers, policymakers, clinicians and health prevention and control personnel find appropriate hotspots and advances.
Materials and methods
Starting from 14 July 2024, we use the Database Science Citation Index Expanded (SCIE) via the Web of Science (WoS) Core Collection provided by Thomson Reuters.
Materials
Microsoft Excel and Matlab are used for data analysis; VOSviewer, Origin and Microsoft Excel are used for data visualisation and chart making.
Eligibility criteria
All included vaccine-related studies from the last 10 years identified as highly cited by Clarivate Analytics. The document types for highly cited papers include regular scientific articles, review articles, proceedings papers and research notes. Letters to the editor, correction notices and abstracts are not counted.10 However, previous studies16 17 were typically limited to articles and reviews, excluding non-article type documents such as meeting abstracts, editorial materials, proceedings papers, letters, book chapters, news items, corrections and notes. We believe that highly cited papers are a hot topic in a certain field, although some papers are non-article type, they have also received a lot of attention and are of great significance for research. Therefore, we do not impose any restriction on the literature types of 3258 highly cited papers (including 18 book chapters and nine processing papers). The 11 early access papers retrieved were of either article or review type and were all included in the study.
Data collection and analysis
We have successively adopted three retrieval methods for data collection. All data are from WoS Core Collection (SCIE), and the retrieval time is 2024-07-14. (1) TS=(vaccine) OR TS=(vaccines): 351 980 studies were included in SCIE, covering a period from 1900 to 2024; (2) (TS=(vaccine)) OR TS=(vaccines)) AND DOP=(2014-01-01/2024-07-14) refined by highly cited papers, totaling 879 414 citations by Clarivate Analytics; and (3) DOP=(2014-01-01/2024-07-14) refined by highly cited Papers, resulting in 182 126 highly cited papers identified by Clarivate Analytics in the same period.
For the data that needs to be visually analysed, we select Tab Delimited File data type, select Full Record and Cited References in Record Content, export the required data with ‘Comma Separated Values’ (CSV) formats, and make charts with Microsoft Excel and VOSviewer. The Journal Citation Reports are used to determine Journal Impact Factors and journal categories.
After collecting the above data, we calculate according to the following formula: (1) Annual Growth Rate=Current Year Total Number of Articles - Previous Year Total Number of Articles) / Previous Year Total Number of Articles18; (2) The calculation formula of average growth rate is as follows:  . We also used Matlab software to perform piecewise fitting for all SCIE papers in the field of vaccines and used the polynomial fitting method for the first half of the data (mainly from 1904 to 1943). Gaussian fitting method was used for the second half of the data (mainly from 1944 to 2023).
. We also used Matlab software to perform piecewise fitting for all SCIE papers in the field of vaccines and used the polynomial fitting method for the first half of the data (mainly from 1904 to 1943). Gaussian fitting method was used for the second half of the data (mainly from 1944 to 2023).
Data reliability
All data were directly exported and collected by WoS Core Collection, and the required calculations were completed by Microsoft Excel and Matlab. After data collection, Huilin Cao and Meixin He verified all obtained data. All data were checked three times by three different authors (Runfeng Shi, Huilin Cao and Meixin He) independently.
Results
Publication trends
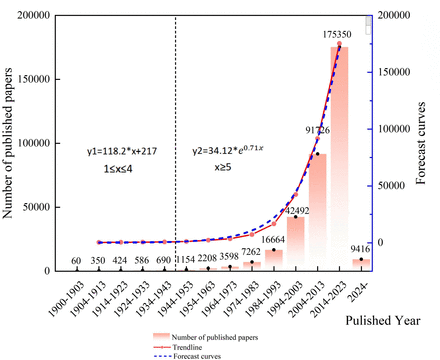
Overall publication trends: We looked at all SCI papers in the vaccine field from 1900 to 2023 in 10 year segments. Since the first SCI paper in the field of vaccines appeared in 1900, research in the vaccine field has been increasing. Due to the period 1900–1904 being less than 10 years and 2024 not yet concluded, we consider it impractical to include these two sets of data in the statistics. According to the growth, the development of vaccines can be divided into two periods. The first period is a period of gentle growth (1904–1943). The second is the period of rapid growth (1944–2023). According to previous mathematical research ideas,19 20 we used the total number of papers published every 10 years to perform piecewise fitting of the data. To facilitate calculation, the first interval (1904–1913) was set as x=1, the second interval (1914–1923) as x=2 and so on, until the last interval (2014–2023) was set as x=12. Y=118.2x+217 with r2=0.9819 (1≤x≤4) and  with r 2=0.998 (x≥5) (figure 1). The main turning point of growth occurred in 1944–1953, with an increase of 464 articles or 67.25%, while in the previous 40 years, the average increase was only 113.33 articles per decade. This may have something to do with World War II and the advances in scientific development that followed. In the following 80 years, the number of published papers in the field of vaccines increased significantly every 10 years, with an average growth rate of 104.96%, which means that the number of papers and studies doubled every 10 years (table 1). Some studies have found that the average growth rate of the entire WoS database in recent 10 years and 20 years is 3.97% and 3.78% respectively,17 indicating that vaccine-related research is growing in importance and international stature, significantly higher than that in other areas.
with r 2=0.998 (x≥5) (figure 1). The main turning point of growth occurred in 1944–1953, with an increase of 464 articles or 67.25%, while in the previous 40 years, the average increase was only 113.33 articles per decade. This may have something to do with World War II and the advances in scientific development that followed. In the following 80 years, the number of published papers in the field of vaccines increased significantly every 10 years, with an average growth rate of 104.96%, which means that the number of papers and studies doubled every 10 years (table 1). Some studies have found that the average growth rate of the entire WoS database in recent 10 years and 20 years is 3.97% and 3.78% respectively,17 indicating that vaccine-related research is growing in importance and international stature, significantly higher than that in other areas.
Overall trends and fitted equation of Science Citation Index Expanded (SCIE) vaccine papers development in 1900–2024.
Growth rate and number of articles published in 1900–2024
Highly cited paper publication trends
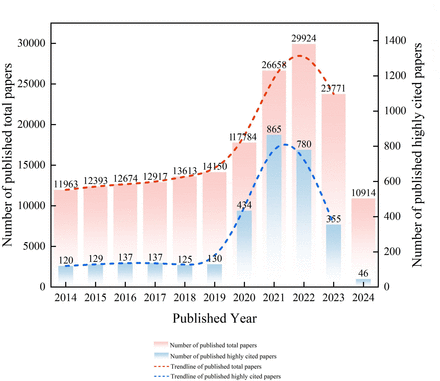
A total of 3258 highly cited papers were published in the vaccine field included in the study. Just as vaccine research began in the 19th century, the number and research direction of highly cited papers in the vaccine field in the recent 10 years were significantly affected by the COVID-19 pandemic. In the 6 years before the emergence of COVID-19 (2014–2019), the number of highly cited papers published in the vaccine field was only 778, with an average of 129.67 highly cited papers per year, and the annual number of highly cited papers was stable without significant fluctuation. After the outbreak of COVID-19 at the end of 2019, the number of highly cited papers increased significantly in 2020 (n=434), an increase of 223.85% compared with the previous year, and reached 865 and 780 in 2021 and 2022, exceeding the total number from 2014 to 2019. From 2020 to 2024, highly cited papers in the field of vaccine research increased significantly, accounting for 76.12%. As the COVID-19 epidemic receded, the number of highly cited papers decreased rapidly. In 2023, the number of highly cited papers was only 355, a decrease of 54.49%. It is expected that the number of highly cited papers will further decrease in 2024. This may due to the fact that the prevention and control of COVID-19 has become a top priority around the world, and the focus of global medical research has shifted towards COVID-19 during the pandemic.8 ,21 We also found that total vaccine-related SCI papers showed a continuous growth before COVID-19 outbreak; thus, we indicate that although vaccine-related papers may decrease in the next few years due to the recession of COVID-19 epidemic, but it will carry on this trend later. Detailed data are shown in figure 2.
Published trends of highly cited and total vaccine papers in recent 10 years.
Countries, institutions and funding agencies
Countries
A total of 157 countries and regions participated in the study of highly cited articles. The top five countries in the number of published articles are the USA (n=1 73 3530.19%), UK (n=64 0190.64%), China (n=54 2160.64%), Germany (n=3,149.64%) and Canada (n=2,628.04%). The USA not only published the most highly cited papers but also cooperated with other countries the most (Total Link Strength:3,043), followed by UK (total link strength: 2308) and Germany (total link strength: 1643). China (total link strength: 1145) and Canada (total link strength: 1262) published more papers but cooperated less with other countries, indicating that they prefer to cooperate with their own authors to write papers.
In addition, we creatively introduced ‘% of highly cited Papers’ and ‘% of funded Papers’. Among them, ‘% of Highly cited Papers’ can reflect the overall research quality of the country’s papers from the side; ‘% of funded Papers’ can indicate a country’s funding skew for vaccines (table 2).
Top 15 most productive countries for highly cited vaccine researches
Interestingly, the top five countries in terms of number of articles also have the largest number of citations and cited times per article, while the ranking of number of articles and total cited times of countries at the bottom are not in one-on-one correspondence. For example, Italy published 197 articles with only 49 078 citations. However, South Africa only published 127 articles, but had 61 881 citations.
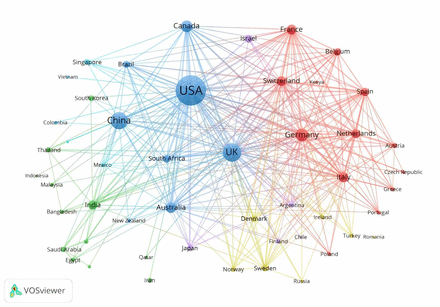
To further understand the cooperation between countries, the visualisation analysis diagram of national cooperation research is shown in figure 3, which only shows the top 50 countries in terms of the number of publications. The visualisation network map of countries contained 50 nodes, 1053 links and six clusters, in which USA, UK, Germany and Canada were located at a central position of the cooperating clusters. This illustrated that authors from these countries prefer to collaborate across national borders.
Network visualisation map for country collaboration in vaccines. A minimum of 22 documents per country was set as the threshold, and 50 countries were included in the map. The thickness of the link between any two countries indicate the extent and intensity of collaboration.
Institutions
We analysed the institutions involved in 3258 highly cited papers. We found a total of 7133 institutions participating in highly cited papers. An average of 2.19 institutions collaborated on each paper, indicating that high-impact research is inseparable from inter-institutional collaboration.
We studied the 15 institutions with the most publications (table 3), which are all from the USA (n=10) and the UK (n=5), and these two countries are also the two countries with the most publications, indicating that the institutions in these two countries have the most important influence in the field of vaccine research. This provides a reference for researchers to choose research institutions for scientific research collaboration. In addition, we also introduced h-Index in our research on institutions, which provides researchers with the dimension of ‘time’ based on citations, and institutions with high influence and long history usually have a higher H-index.
Top 15 most productive institutions for highly cited vaccine researches
Funding agencies
Among the 3258 highly cited papers included in the study, there were 4787 funded institutions in total, of which 890 papers (27.32%) were not funded by any institution. Highly cited funded papers received 2.02 institutions’ funding on average. The higher the number of highly cited papers funded by funding institutions, the more important it is in this field. We found that funding for vaccine research involves not only national sectors such as the US Department of Health Human Services and the National Natural Science Foundation of China, but also private companies such as The Wellcome Trust and Pfizer and international partners like the European Union. The top five funded institutions with the largest number of highly cited papers are the US Department of Health Human Services (n=80 7240.77%), National Institutes of Health USA (n=76 8230.57%), National Institute of Allergy and Infectious Diseases (n=2,93 80.99%), UK Research Innovation (n=2,628.04%) and National Natural Science Foundation of China (n=2,226.814%). The data of the TOP15 institutions with the most funded papers are shown in online supplemental table 1.
Supplemental material
Authors and researches
A total of 30 926 authors were retrieved from 3258 highly cited papers in the field of vaccines, with an average of 9.49 co-authors per paper. We found that most authors (n=23 586, 76.27%) only published one highly cited paper. There are about 228 authors (0.74%) who published 10 or more papers; all these authors published 1041 highly cited papers, accounting for 31.95% (1041 to 3258), they involved 364 799 citation times, accounting for 41.48% of the total citation times. In addition, we also collected the detailed information of the authors with more than 30 papers and innovatively introduced the number of papers with major contributions (ie, only the papers of the first and last authors were considered) (online supplemental table 2). The cooperative network map of authors contained 50 nodes, 169 links and 10 clusters, in which TH Keipp, M Laurie and K Florian were located at a central position of the cooperating clusters. What’s more, we have labelled the nationalities of the top 15 most productive authors as representatives in online supplemental figure 1 to provide a more visual understanding of the transnational collaboration among authors.
Supplemental material
Supplemental material
We also found that a total of 342 group authors participated. Of the top 15 groups with the most highly cited papers, six are named after COVID-19 (Oxford COVID Vaccine Trial GRP, COVID-19 Genomics UK COG UK CONSOR, Overcoming COVID-19 Investigators, 2019nCOV 302 Study GRP, Accelerating COVID-19 Therapeutic, CDC COVID-19 Respons Team), accounting for 40%, indicating that the COVID-19 pandemic has had a profound impact on global vaccine research priorities and directions. Multiple research groups have been established in a short period of time, demonstrating the importance of COVID-19 to policymakers, clinicians and researchers around the world.
Among 3258 highly cited papers, 2951 OA articles were found, accounting for 90.58%, indicating that the extensive readability of OA articles makes it easier for papers to become highly cited papers. Further research found that 2951 OA articles were cited for a total of 800 389 times, with an average of 271.23 times per article. A total of 307 non-OA articles were cited for 79 605 times and 259.30 times on average. In addition, only one non-OA article is included in the top 15 cited articles, indicating that OA articles are more likely to get more cited times.
Based on the analysis of the literature types of 3258 included studies, we found that there were 2168 articles and 1090 reviews, while the vaccine field published 131 692 articles during the same period (2014–2024), accounting for 1.65%; published 27 796 reviews, accounting for 3.92%. The proportion of highly cited papers in review is more than twice that in article, indicating that review is more likely to become highly cited papers.
We also focused on the top 15 cited papers, using data including journal name, publication date, WoS categories, number of citations and overall citation rate (total citations/article age). Previous studies have found that the number of citations in an article increases with time, and even the top articles have no citations at the time of publication. Therefore, the calculation method of ‘Overall citation rate’ can be adopted to effectively exclude the influence of time.22 In addition, we believe that since the data are all from highly cited papers in the recent 10 years, there will be a large error if the calculation is simply based on ‘year’. Therefore, we use ‘month’ to calculate (eg, January and December 2023 are simply classified as 2023, so the two articles are calculated as 1 year by year, but 19 and 8 months by month, the difference is nearly two times), which can effectively avoid error and find more excellent and important papers more accurately. Detailed results are shown in table 4.
TOP 15 articles with most citations for highly cited vaccine researches
Journals and languages
3258 highly cited papers were widely published in 735 journals, with an average of 4.43 articles per journal. Only one article was published in 391 journals, accounting for 53.20%. There were 69 journals with more publications and greater influence in the field of vaccines (n≥10, ie, more than one highly cited paper per year on average); highly cited papers reflect the most influential papers in a certain field, and the journals that publish the most highly cited papers are more influential and recognised in this field. Table 5 lists 15 journals with the largest number of publications. The introduction of index ‘Percentage of Related articles’ can facilitate scientific researchers to select journals with higher acceptance probability and greater influence in the vaccine field when publishing articles in the vaccine field. The percentage of related articles in the top five journals is relatively low, with the highest being only 3.56%. However, these five journals are all traditional prestigious journals, indicating that publishing in traditional prestigious journals has a higher chance of being discovered and seen, resulting in higher citation rates.
Top 15 Journals with most researches for highly cited vaccine researches
By analysing the writing language of 3258 highly cited papers retrieved, we found that all 3258 highly cited papers were written in English. A comparison of all the publications in the SCIE database in the vaccine field in the recent 10 years shows that the most publications are written in English (n=18 292 6980.93%), followed by Spanish (n=5270.29%), German (n=5120.28%) and French (n=3640.20%), and the other 19 commonly used languages account for less than 0.1%. Highly cited papers must first be read by researchers around the world. The widespread use of English in the world gives birth to highly cited papers. In the field of vaccine research and application, it is essential to write highly cited papers in English.
Research areas and co-occurrence keywords
Within the time period 2014–2024, 3258 highly cited vaccine research papers mainly involved 90 research directions (WoS categories). The top five research directions are General Internal Medicine (n=51 4150.78%), Science Technology Other Topics (n=47 5140.58%), Immunology (n=34 0100.44%), Research Experimental Medicine (n=32 9100.10%) and Public Environmental Occupational Health (n=2,979.12%). In addition to basic medicine, clinical medicine and preventive medicine, the Vaccine Research also involves non-medical research topics such as Materials Science (n=692.12), Engineering (n=300.92), Physics (n=300.92) and Mathematics (n=120.37), as well as interdisciplinary fields such as Mathematical Computational Biology (n=100.31) and Medical Informatics (n=90.27).
Authors’ Keywords and Keyword Plus (which provided additional search terms from the titles of articles cited by the Authors in their bibliography and footnotes) provide a useful basis for other researchers to accurately locate their research, and Keyword Plus is just as valid as authors‘ keywords,23 and co-occurrence keywords show the focus of vaccine researchers. We found that in 3258 highly cited papers, the authors provided 3970 co-occurrence keywords and 6674 Plus keywords, and 9848 co-occurrence keywords were obtained after excluding repeated co-occurrence keywords, with an average of 3.02 co-occurrence keywords per article. The 15 co-occurrence keywords with the highest frequencies were COVID-19, infection, vaccination, immunogenicity, United States, Dendritic cells, virus, efficacy, immunotherapy, safety, immune responses, immunity, disease, cancer and T cells. These high-frequency co-occurrence words reveal the main hotspots of current vaccine research in the world, mostly focusing on viruses and immunisation. The most frequent co-occurrence words are COVID-19 (including SARS-CoV-2, 2019-COVID and SARS2), which appeared in 1720 highly cited studies. This indicates that due to the global pandemic of COVID-19, resources in the vaccine field have been skewed towards COVID-19 in the recent 10 years due to the need for disease prevention and control.
To further explore the co-occurrence relationship between keywords, we selected the 50 keywords with the highest number of mentions and created a visual map, which can be seen in online supplemental figure 2.
Supplemental material
Discussions
Future trend prediction
As mentioned earlier (Publication Trends), current research in the field of vaccine is in high gear. We believe that vaccine-related researches will continue to grow rapidly over the next decade. What’s more, because of the irreplaceable role of vaccines in public health,24 with the development of science and technology, there will be new ideas and progress in the field of vaccines, such as a wider range of animal and plant vaccine applications. In addition, the COVID-19 epidemic has spawned many new technologies for vaccine research.25 The safety, protective effects and side effects of the vaccine on humans cannot be evaluated completely without medium- and long-term follow-up studies. The systemic effects caused by COVID-19 cannot be ignored,26 and the long-term effects of vaccination or not on the patients require continuous follow-up studies. Collectively, in spite of the recession of COVID-19 epidemic caused the decrease of vaccines’ highly cited researches, we believe that highly cited papers and total SCI papers in the field of vaccine will decrease to a certain point above pre-epidemic level and remain stable growth until next epidemic outbreak or revolutionary technologies occur.
What caused South Africa gaining the highest average citation rates among the top 15 most productive countries?
In section ‘Countries, Institutions and Funding Agencies’ subsection ‘Countries’, we mentioned that South Africa has a high average citation frequency, even though it does not have an outstanding number of publications. We believe this may be due to the fact that in November 2021, a new COVID-19 variant was first monitored by a genomic surveillance team in the South Africa and Botswana, which was detected in 87 countries and territories in the next 3 weeks, leading to rapid transmission and pandemics in areas with high levels of immunisation of the populations (who had been vaccinated with COVID-19 vaccines).27 This can be corroborated by the fact that the three most cited papers published in South Africa, ‘Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralisation’, ‘mRNA-based COVID-19 vaccine boosters induce neutralising immunity against SARS-CoV-2 Omicron variant’ and ‘Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression’, received 760, 756 and 752 citations, respectively, ranking 9, 10 and 11 in total 150 highly cited papers about Omicron variant. What’s more, the quality of articles (eg, Argentina, USA, UK, Brazil, Germany, Turkey and South Africa shared an article with 10 034 citations, entitled ‘Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine’) and regional environment (including sanitary conditions and epidemic conditions, which means more infectious diseases and sample data for research).28 Finally, the average is more susceptible to extremes of data due to the lower volume of publications of South Africa. All of the above factors enabled South Africa a significantly higher average number of citations than any other country in the top 15 most productive countries.
Limitations of this research
We retrieved 3258 highly cited papers in the WoS core collection, since the large amount of data in the article, it is impossible to check and compare article by article, so there may be some deviation in our research results. For example, in the statistics of countries and institutions, due to the large amount of data, it is impossible to check the attribution of the authors in all these papers (including countries and institutions), so the information of all authors in each article is included in the statistics. 30 926 authors are involved in 3258 highly cited papers, and the average number of authors in each paper is 9.49. Germany published 314 papers with 7795 authors, with an average of 24.82 authors per paper, 2.6 times the average. Cross-country collaboration was not included in the study due to the large number of data. However, it is indisputable that the excessive number of coauthors in some countries may cause interference to the ranking of countries and institutions.For an example of this, the paper ‘Live attended vaccine sCPD9 elicits superior mucosal and systematic immunity to SARS-CoV-2 variants in Hamsters’ involves 35 authors from 18 different German institutions, two Chinese institutions and one Cyprus institution). The reason for having a large number of authors in an article may be due to the complexity of experimental research and the need for collaboration among multiple people due to the large amount of data. Future bibliometric research requires the use of big data algorithms or artificial intelligence to quickly read and accurately analyse large quantities of articles.
Strengths of this research
As far as we know, most of the bibliometric studies in the vaccine field in the past focused on a certain pathogen or disease or a single vaccine, or only analysed 100 highly cited papers in the vaccine field. There was a lack of bibliometric analysis on the overall output of global vaccine research and application papers and highly cited papers in vaccine research. Using bibliometrics to analyse the global vaccine research field from 2014 to 2024 can better grasp the status of this field in international research. We found that with the outbreak of the World War II and the rapid development of global economy and science and technology after the war, global vaccine research received great attention. And more scientific research resources, especially scientific research funds, began to favour vaccine field. The most significant performance is that during the 80 years from 1944 to 2023, the average growth rate of vaccine-related SCI papers per decade reached 104.96%. With the COVID-19 pandemic, high-quality vaccine research papers have seen a rapid increase, with the proportion of highly cited papers published in the 3 years of the pandemic (2020–2022) reaching 63.81% from 2014 to 2024. In addition, compared with the study that only analysed highly cited papers, we used the WoS database to comprehensively analyse and screen out highly cited papers in the recent 10 years, focusing on the latest research direction, which has better timeliness. It also excludes some of the older basic research papers that, despite their importance, are now common knowledge in the field and far removed from the frontiers of medicine.
Compared with previous studies,29 this study conducted a multidimensional analysis for all highly cited papers retrieved and presented a more comprehensive overview of global vaccine research hotspots, providing a powerful reference for researchers engaged in vaccine research and design to collect literature and conduct intelligence research. We also innovatively introduced the index of ‘number of papers with major contributions’, which can only consider the first author (or the second, third or co-first author) and the last author (including the corresponding author or co-corresponding author) in the statistics, which can effectively describe the contribution of a certain author in the field, facilitate the determination of the main research direction of the author and effectively eliminate the interference of mutual ‘pseudonymous’ papers. The introduction of ‘co-occurrence keywords’ can fully show the focus of vaccine researchers and help quickly grasp the main hotspots of vaccine research from 2014 to 2024. This study is also conducive to the determination of newly selected institutions and research fields, as well as the prediction of future research hotspots, so as to guide CDC to formulate correct public health policies and provide a more relaxed policy environment and more powerful policy support for vaccine research and development.30 31
In this study, polynomial fitting and Gaussian fitting methods were used to fit the growth of vaccine-related research over the past 120 years since 1904, and the constructed curve provided reference and prediction for the future development of vaccine research. The global COVID-19 pandemic from 2019 to 2022 and the development of new technologies in recent years have led to more people getting vaccinated,32 33 which means that the previously severe public vaccine hesitancy has been improved.34 35 With the emergence of new technologies, the field of vaccine research is bound to experience even more vigorous development.
Conclusion
This paper presents a panoramic view of global vaccine research and application and summarises the overall trend of global vaccine research and application papers published. In fact, this study can help CDC officials, clinical doctors and vaccine developers better grasp the overall research situation in the global vaccine field,36 providing targeted assistance for public health policy formulation and future research, such as how to choose appropriate journals to publish their vaccine research results, how to choose influential authors and institutions for scientific research cooperation and providing reference guidance for newcomers or students who want to engage in vaccine research in the future. Governments and decision-makers of various countries can use this research to identify the most influential countries and institutions in the global vaccine research and application field to send them for exchange and learning, strengthen cross-border scientific and technological cooperation in vaccine research and focus on solving problems that arise in the process of vaccine research and application. Of course, bibliometric analysis may also help vaccine researchers grasp and predict new trends in vaccine development and design, promoting high-quality development of vaccine research and application.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
Many thanks to Professor Xianbo Wu for her continuing support and suggestions throughout the process of writing this paper. We are very grateful for the support provided by Ms. He Zhiqing from South China Normal University in mathematical method and data reviewing.
References
Footnotes
Contributors All authors contributed to the study conception and design. Theme determination, data collection, method application, article writing, reviewing and editing were performed by RS, HC and MH. Reviewing and suggestions were performed by CY, YW, XW, YZ and LL. Theme determination, conceptualisation, methodology, investigation, reviewing and editing were performed by XX. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Professor XX is the guarantor of this manuscript.
Funding This work was supported by Guangdong's 2014 Education and Research Major Subject[Grant number 2014JKZ0008].
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.