Article Text
Abstract
Objective Physical activity (PA) has been generally recognised as beneficial for health. The effect of a change in PA on kidney biomarkers in healthy individuals without kidney disease remains unclear. This manuscript synthesised the evidence of the association of changes in PA with kidney biomarkers in the general population free from kidney disease.
Design Systematic review and meta-analysis.
Data sources Embase, PubMed, MEDLINE and Web of Science databases were searched from inception to 12 March 2023.
Eligibility criteria for selecting studies Studies of longitudinal or interventional design were selected initially. The following studies were excluded: (1) case-control studies, (2) studies where PA was measured at a single time point, (3) populations with known kidney disease, (4) studies evaluating the impact of a single episode/event of PA and (5) non-English language studies.
Data extraction and synthesis Two independent reviewers extracted data from a pre-designed table and assessed the risk of bias using the Cochrane Risk of Bias tool. Data were pooled using a random-effects model. Hedge’s g was used to synthesise effect sizes and obtain an overall estimate. Heterogeneity between studies was measured using I2. Funnel plots and Egger’s test were performed to evaluate the risk of biased results.
Results 16 interventional studies with randomised or non-randomised designs involving 500 participants were identified. The median follow-up was 84 days. 10 studies were at high risk of bias. Studies with low quality were published prior to the year 2000. Changes in PA were found only to have a positive association with serum creatinine (SCr) (Hedge’s g=0.69; 95% CI 0.13, 1.24; I2=81.37%) and not with plasma renin activity (PRA), urea, or urine albumin-to-creatinine ratio (UACR). The positive association was only observed in people with obesity and those who exercised for more than 84 days.
Conclusions Higher levels of PA are associated with increased SCr levels in healthy people. It remains unclear if this association is related to impaired kidney function or gain in muscle mass, as data on other kidney biomarkers did not support a certain link.
PROSPERO registration number This review has been registered on PROSPERO (CRD42023407820).
- Exercise
- Meta-Analysis
- behaviour
- Nephrology
- Physical Fitness
Data availability statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Different from existing studies that mainly focus on people with kidney diseases, this study focuses on populations without known kidney diseases, therefore extending the current knowledge.
This study analysed important kidney biomarkers, including serum creatinine, urine albumin-to-creatinine ratio and plasma renin activity, providing a broader understanding of the possible effect of changes in physical activity on kidney health.
Constrained by small sample sizes and heterogeneity, several included studies had limited population sizes and high attrition rates, which may lead to biases, particularly concerning the observed heterogeneity in outcomes.
Background
Cardiovascular disease (CVD) is a major global health issue, causing approximately 17.9 million deaths annually, or 32% of all global fatalities as of 2019.1 CVD also imposes a significant economic burden on healthcare systems worldwide.2 3 Physical inactivity has been recognised as a risk factor for CVD events,4 5 while performing physical activity (PA) is beneficial to the prevention of CVD, along with other chronic conditions such as chronic kidney disease (CKD).6
Despite the myriad of benefits of PA on cardiovascular health, its effect on kidney function is not well established. Impaired kidney function is a risk factor for CVD;7 it is plausible that PA might also positively affect kidney function.8 Serum creatinine (SCr)-based estimated glomerular filtration rate is commonly used in clinical practice, and creatinine is a product of muscle metabolism.9 Therefore, any effect of PA on muscle metabolism may indirectly affect the measurement of kidney function. There is also evidence suggesting that extreme levels of PA may induce kidney damage via rhabdomyolysis or dehydration.10
Evidence from randomised controlled trials suggests that PA is associated with multiple metrics of kidney function. However, the evidence is controversial. PA is inversely associated with the risk of kidney function decline in people aged over 65, with an average estimated glomerular filtration rate (eGFR) of around 80 mL/min/1.73 m2.11 Yet, the same association was not observed in a younger general population (aged 26–65 years) with a much higher average eGFR of 108 mL/min/1.73 m2.12 Studies assessing PA intensity include data showing that accelerometer-measured low- and moderate-intensity PA is positively associated with eGFR in a general Japanese population (aged 35–79 years, average eGFR 92.6 mL/min/1.73 m2) across sexes and ages.13
PA has also been linked to urinary albumin excretion. As the dysfunction of the kidney endothelial barrier and atherosclerosis contribute to the leakage of albumin into the urine, microalbuminuria has been suggested as an indicator of kidney endothelial dysfunction.14 The association between high PA levels and lower microalbuminuria has been observed consistently across variant populations.15 Novel biomarkers of kidney impairment, such as liver-type fatty acid-binding protein, have also been found to be negatively impacted by habitual physical activities.16 The degree of stress on the proximal tubule may be attenuated through PA, regardless of the kidney’s functional reserve, suggesting PA’s health benefits on the kidney structure.
Although the effects of PA on the kidneys have been studied, many articles focus on the acute effect of PA, and they are not instructive of the impacts of changing habitual PA. The study population is often restricted to patients with CKD/end-stage kidney disease, including those who undergo dialysis. These research findings may not be applicable to the general population without known kidney disease. A number of intervention studies discussed the effect of PA in combination with other treatments, such as diet and pharmaceutical approaches; thus, it is difficult to measure PA’s direct effect. To date, there is a lack of systematic reviews of the literature that have been conducted on the effect of changes in PA on kidney health in populations without pre-existing kidney diseases. In this context, this study aimed to conduct a systematic review and meta-analysis to bridge the knowledge gap.
Methods
This review has been registered on PROSPERO (CRD42023407820). In this review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement17 and the Cochrane Handbook for Systematic Reviews of Interventions.18 We followed the Population, Intervention, Comparison, Outcomes and Study framework to develop our search strategy.19 To be specific:
Population: Adults without known kidney disease.
Intervention: PA.
Comparator: Kidney-relevant biomarkers before PA.
Outcome: Changes in kidney-relevant biomarkers after PA.
Study Design: Cohort or interventional study.
The research theme is: ‘In adults without known kidney disease, to what extent does PA affect the levels of kidney-relevant biomarkers compared with baseline levels?’
One author (QL) systematically searched Embase, PubMed, MEDLINE and Web of Science databases from inception to 12 March 2023. The inclusion criteria were (1) cohort studies or interventional studies (randomised/non-randomised), (2) studies in which the duration and/or intensity of PA was measured at least twice and (3) studies in which the study population is based on the general population. It is expected that people with CKD will form around 10% of the general population, so we additionally extracted baseline eGFR and urine albumin-to-creatinine ratio (UACR) (where available) as an indicator of the baseline level of kidney function in these studies. We also carefully examined the description of the study population in selected studies. Studies that met the following criteria were excluded: (1) case-control studies, (2) studies in which PA was measured at a single time point, (3) studies conducted specifically in populations with pre-existing kidney diseases, such as CKD, dialysis and kidney transplantation, (4) studies evaluating the impact of a single episode of PA, such as a sporting event, and (5) studies that were not published in English. The detailed search terms can be found in the supplemental materials (online supplemental table S1).
Supplemental material
Two authors (QL and PW) independently decided which studies should be included in this study, and any disagreements were resolved through a discussion with two other authors (CC and PM). To maximise the coverage of sources, one author (QL) checked the references of the selected articles and evaluated their relevance after reading the full text. Additively, one author (QL) performed manual searches for relevant studies.
Study exposure
The study exposure was the change in PA. The change in PA was denoted as a categorical variable, that is, from being sedentary to being active.
Study outcome
The primary study outcome was the change in kidney-relevant biomarkers, including but not limited to SCr, cystatin C and UACR. This change was defined as the difference in a biomarker’s level after the completion of a change in PA, for example, the difference in SCr levels before and after a 12-week aerobic exercise programme. In addition, long-term kidney outcomes, such as the first diagnosis of CKD and the presence of microalbuminuria, were also collected if relevant literature was identified.
Quality assessment
All the selected studies were interventional studies. The risk of bias for each selected study was assessed using the Cochrane Risk of Bias tool20 by two reviewers independently. Seven domains of bias (sequence generation, allocation concealment, blinding of participants and personnel/outcome assessment, incomplete outcome data, selective reporting and other biases) were assessed. The overall risk was categorised as low, high, critical, unclear or no information. A study was biased if the loss to follow-up was 20% or above.21 Any disparities in judgement raised between the two reviewers were resolved through discussion with the help of a third author as needed.
Data synthesis
Using a pre-designed table, information was extracted on the first author’s family name, publication year, study type, study location, baseline characteristics of exercise groups, type of exercise, length/frequency/intensity of exercise and outcomes. In case a study has both exercise and sedentary groups, only the information of the group that performed PA was included to align with our research theme.
For studies with multiple measurements, we used the baseline and the final measurement to calculate the change. For example, if a kidney biomarker was measured at exercise week 0 (the baseline week), week 3 and week 6 (the final week), the change in the biomarker between week 0 and week 6 was used. In cases where subgroup findings were reported, those findings were extracted and compiled for meta-analysis, subject to data availability. The median (IQR) of reported data was converted to the mean (Standard Deviation [SD]) following established methods.22 In case the standard error of the mean (SEM) was provided only, the SD was calculated from SEM multiplied by the square root of the number of studies.
As between-study heterogeneity was anticipated, we constructed random-effects models23 to combine the mean (SD) of selected studies and applied the inverse variance weighting method. The Hedge’s g expresses the difference of the means in units of the pooled SD; it measures the effect size for the difference between the means. This study used it to synthesise effect sizes and obtain an overall estimate of the effect of PA. It incorporated a correction factor for small sample sizes, which is useful as many PA interventions were of small scale.24 For interpretation, values of 0.2, 0.5 and 0.8 were regarded as small, medium and large effects, respectively.25 Heterogeneity between studies was examined using the I2 statistic, and an I2 above 50% means substantial heterogeneity.26 27 Subgroup analyses and meta-regressions were conducted to investigate heterogeneity across age, obesity and length of exercise. Due to insufficient data, some subgroup analyses and regressions were not performed for all outcomes. Funnel plots and Egger’s test were performed to evaluate the risk of biased results.28 Statistical analyses were performed using STATA 17 (StataCorp, USA). Data were visualised using Robvis (https://mcguinlu.shinyapps.io/robvis/).29
Sensitivity analysis
Leave-one-out analysis was performed to identify influential studies by conducting the meta-analysis multiple times while removing one of the included studies during each iteration. Results were presented as leave-one-out figures. A cumulative meta-analysis was also performed for each outcome according to publication year to identify secular trends.
Patient and public involvement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting or dissemination plans of our research.
Results
Identification of studies
After removing duplicated studies, 10 294 potentially relevant studies were identified. Initial screening based on title and abstracts resulted in 155 studies retrieved for further evaluation. Following the full-text assessment, 150 studies were excluded, leaving 5 studies. Additionally, 20 studies were identified through hand search and reading citations, of which 9 were excluded, leaving 11 studies. In total, 16 studies were included for further analysis (online supplemental figure S1).
Characteristics of the included studies
All the 16 included studies were of interventional design. The duration of intervention ranged from 1 month to 9 months, with a median duration of 2.8 months (11 weeks). The identified 16 studies have a total study population of 500 people (ranging from 4 to 162 individuals). The average age of participants was 50.1 years. 10 studies recruited people with essential hypertension,30–39 1 study recruited people with type 2 diabetes mellitus,40 2 studies recruited people with obesity,41 42 2 studies recruited healthy people43 44 and 1 study recruited patients with heart failure.45 Participants in 12 studies were required to perform aerobic exercise only30–39 44 45; 2 studies involved aerobic exercise and its combinations with strength training,40 42 and 2 others involved resistance training only.41 43 All the studies have a similar exercise frequency of three to five sessions per week, while the length of sessions varies according to the exercise intensity, with a median of 12 weeks. Seven studies have an attrition rate of 20% or above,30 31 33 38 39 41 42 with a maximum of 30.9%.33
Eight studies used maximum oxygen uptake (VO2max) to measure the intensity of aerobic exercise,30 32 36–39 44 45 with a few studies using heart rate reserve,33 maximum heart rate34 35 42 and lactate threshold.31 40 For resistance and strength training, repetition maximum was used to measure the exercise intensity (online supplemental table S2).
Measurement of physical activities
14 studies required participants to perform on-site PA under close supervision. The low-workload group in the study by Hagberg et al30 was supervised for the first month and relied on self-reported forms for the remaining 8 months. All the participants in the study by Passino et al45 had self-conducted exercises with their compliance to the instructions checked at the beginning and near the end of the study. All the studies have reported the arrangement of physical training.
Measurement of the outcomes
SCr, plasma renin activity (PRA) and urea were the most measured biomarkers in selected studies. Two studies reported eGFR,36 42 one study42 reported urine albumin-to-creatinine ratio (UACR) and one study31 was on angiotensin II (Ang II). Twelve of these studies measured fasting biomarkers, while 3 studies have not specified the fasting status42 44 45; 1 study explicitly measured biomarkers after participants had ‘a light breakfast’.39 All the biomarkers were measured under resting conditions.
Potential bias and quality assessment
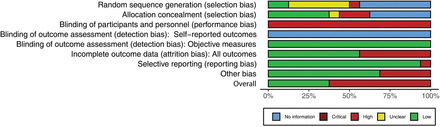
Overall, the selected studies have medium to low quality. Ten out of 16 studies were evaluated as having a high risk of bias. Most studies had a less representative cohort, especially those published decades ago, as early as in the 1980s.30 31 Studies with better population representation were published after 2000.41 42 The incomplete outcome data (attrition bias) were another major source of inferior quality, with seven studies having a high attrition rate of over 20%.30 31 33 38 39 41 42 It was impossible to blind participants in supervised situations due to the nature of the PA as an exposure. Considering the nature of the intervention design and the objective evaluation of outcomes through laboratory testing, all studies have a high risk for blinding of participants and personnel (performance bias) and a low risk for blinding of outcome assessment (detection bias). Six studies with a randomised design have provided information on how the random sequence was generated.30 38 40 42 43 45 Some studies also have a higher risk of measurement error for exposure and outcome (online supplemental table S3, figures 1 and 2).
Summary of the risk of bias by the Cochrane Risk of Bias tool.
Study-specified risk of bias using the Cochrane Risk of Bias tool.
Changes in physical activity and serum creatinine
The meta-analysis included six study populations from four studies,33 41–43 including 197 participants with an average PA duration of 73 days. The majority of findings have a mean Hedge’s g on the right side of the reference line with a wide 95% CI. The pooled result showed a moderate positive effect of PA on SCr (Hedge’s g=0.69; 95% CI 0.13, 1.24). Substantial heterogeneity was detected among cohorts (I2=81.37%) (figure 3a).
Meta-analysis on the associations of changes in physical activity (PA) with kidney-relevant biomarkers: (a) serum creatinine; (b) estimated glomerular filtration rate; (c) urine albumin-to-creatinine ratio; (d) plasma renin activity; (e) urea. Note: Szulinska et al:42 patients received endurance training. Szulinska et al:42 patients received both endurance and strength training. Zaman et al:41 patients with obesity. Zaman et al:41 patients without obesity. Hagberg et al:30 patients performed low-intensity PA. Hagberg et al:30 patients performed moderate-intensity PA. Matsusaki et al:38 patients performed low-workload PA. Matsusaki et al:38 patients performed high-workload PA. de Oliveira et al:40 patients performed aerobic training. de Oliveira et al:40 patients performed strength training. de Oliveira et al:40 patients performed aerobic and strength training. Zaman et al:41 patients with obesity. REML, Restricted maximum likelihood.
Stratifying by obesity, only two groups of participants with obesity from a single study42 have a statistically significant pooled effect (Hedge’s=0.74; 95% CI 0.29, 1.20). Stratified by the median of the length of exercises (12 weeks), only two cohorts from one study who have undergone exercise over 12 weeks have a significant pooled effect (Hedge’s=0.74; 95% CI 0.29, 1.20) (online supplemental figure S2a,b).
The funnel plot showed mild asymmetry, and Egger’s test showed no small-study effects (p value=0.21). Sensitivity analysis showed a consistent result as that of the primary analysis; the removal of one cohort from Zaman41 largely attenuated the pooled effect (Hedge’s=0.38; 95% CI 0.05, 0.72). Obesity was identified as the only important source of heterogeneity. Cumulative meta-analyses according to the year of publication showed significant evidence of secular trends for SCr (online supplemental figure S2c–e).
Changes in physical activity and eGFR
Three cohorts from two studies, 36 42 which included 50 people with an average exercise duration of 12 weeks, were identified. No significant effect was found in the pooled result of the exercise on eGFR (Hedge’s g=−0.30; 95% CI −0.83, 0.24; I2=48.57%) (figure 3b).
Changes in physical activity and urine albumin-to-creatinine ratio
Two cohorts from one study,42 which included 38 people with an average exercise duration of 3 months, were identified. No statistical significance was found in the pooled result of the exercise on UACR (Hedge’s g=−0.15; 95% CI −0.59, 0.29) (figure 3c).
Changes in physical activity and plasma renin activity
13 cohorts from 11 studies30–32 34–39 44 45 included 184 people with an average exercise duration of 129 days in the meta-analysis. No association between PA and PRA was observed (Hedge’s g=−0.12; 95% CI −0.32, 0.09). Minor heterogeneity was found among cohorts (I2=2.54%) (figure 3d).
In stratified analyses, no associations were found in obesity status, exercise length, and age groups. There was no statistically significant effect of exercise on PRA in people aged 60 and above; the upper 95% CI was close to zero (Hedge’s=−0.54; 95% CI −1.14, 0.06) (online supplemental figure S3a–c).
The funnel plot showed good symmetry. Egger’s test showed no small-study effects (p value=0.39). Sensitivity analysis showed a consistently insignificant result as that of the primary analysis, with no influential single studies. Meta-regression showed no important sources of heterogeneity. Cumulative meta-analysis showed no significant changes in research findings (online supplemental figure S3d–f).
Changes in physical activity and serum urea
Five cohorts from three studies40 41 43 included 48 people with an average exercise duration of 73 days. No association was found between PA and urea (Hedge’s g=−0.15; 95% CI −0.50, 0.20). No heterogeneity was found among cohorts (I2=0.00%) (figure 3e). Data were consistent in subgroup analyses (online supplemental figure S4a).
The funnel plot showed good symmetry, and Egger’s test showed no small-study effects (p value=0.39). Sensitivity analysis showed a consistently insignificant result as that of the primary analysis, with no influential single studies. Meta-regression showed no important sources of heterogeneity. Cumulative meta-analyses showed no significant changes in research findings (online supplemental figure S4b–d).
Changes in physical activity and other kidney-related biomarkers
A study by Kiyonaga et al31 showed that after 20 weeks of mild aerobic exercise, the average level of angiotensin II in eight patients with essential hypertension increased significantly from 58 to 91 pg/mL. However, the increase in angiotensin II was not observed after completing the first 10 weeks of training.
Discussion
In this systematic review and meta-analysis of 16 interventional studies involving 500 people without known kidney diseases, we evaluated the available data exploring the association of change in PA with kidney function. Change in PA was found only to have a positive association with SCr, not with eGFR. There was some limited evidence that participants with obesity and people who exercised for more than 12 weeks may have a larger increase in SCr as compared with their counterparts. Sensitivity analysis was in line with the primary analysis; mild publication bias and a secular trend were found. The general quality of studies was suboptimal to make robust conclusions, and the number and size of studies were generally small (ranging from 4 to 112 participants).
Due to the possibility of PA inducing muscle growth, which is the primary source of SCr, the role of body composition in the association between PA and SCr deserves discussion. Among three studies reporting on SCr and body composition, Szulinska et al42 reported a significant increase in lean body mass and SCr, and a decrease in body fat% in a population receiving endurance and strength training for 3 months; Trabelsi et al43 reported no significant changes in body fat% but a significant increase in SCr in a population receiving 1-month resistance training, while Sikiru et al33 reported no significant change in body fat% and a likely increase in SCr in a population receiving 8 weeks of aerobic training. It is noteworthy that the study populations of the above studies had markedly different body compositions, with the latter two having low baseline body fat%, that is, 11.9% and 13.5%, respectively, while the population in the first study had an average body fat% of over 33%. Additionally, Kinoshita et al36 reported no significant change in eGFR in 12 non-obese people after a 10-week aerobic exercise, which implied a possible insignificant change in SCr. Therefore, the impact of PA on SCr levels may be related to body composition.
In the pooled result, there was no statistically significant association of exercise with UACR (figure 3c). The pooled result came from two cohorts of one study with a small study population.42 It should be noted that while urinary albumin and urinary creatinine both increased after endurance-strength training, their ratio did not change much, implying a balanced increase of albumin and creatinine. In people who only performed endurance training, no increases in albumin, creatinine or UACR were found. Therefore, the increase in albumin and creatinine might be caused by increased muscle mass or post-activity proteinuria.
As an important hormone secreted by the kidney for regulating blood pressure, renin has long been a topic of interest. Among the biomarkers under discussion, renin is the most extensively researched, with the earliest studies dating back to the 1980s. However, 9 of the 11 studies on renin were published in 1992 or earlier, with only 2 published after 2000. The studies involved a small number of participants, with most having between 10 and 20 individuals. Nevertheless, the cumulative meta-analysis based on publication year revealed a progressively narrowing 95% CI with an upper limit approaching zero and a consistently negative effect size. In the research conducted by Matsuaki et al,38 despite the absence of a significant difference in baseline PRA between the low- (50% VO2max) and the high-workload (75% VO2max) group, both cohorts manifested a similar pattern characterised by two interlocking M shapes throughout six measurements conducted at baseline and week 1/2/4/7/10. The PRA pattern exhibited by the low-workload group between week 1 and week 10 was similar to that of the high-workload group between week 0 and week 7. The PRA change in the low-workload group was ‘delayed’ by 1 week compared with the high-workload group. Specifically, the PRA in the low-workload group experienced a slight decline in the first week, followed by an increase in the second week, whereas the PRA in the high-workload group increased in the first week. The underlying mechanism of this finding remains elusive.
Kiyonaga et al31 reported a significant increase in angiotensin II after 20 weeks of mild aerobic exercise in eight patients, yet no significant increase was observed by week 10. As renin secretion is the first step in the production of angiotensin II, it can be speculated that exercise lasting over 20 weeks may significantly impact renin (and thus angiotensin II). Renin is rarely measured in clinical practice and is affected by many antihypertensive drugs. Although these findings are interesting, any effect of PA on renin is unlikely to translate into information used to inform clinical guidelines.
Urea is clinically measured to evaluate kidney impairment, although to a lesser degree than SCr. There was a lack of a significant association between exercise and urea levels. One possible explanation is that considering the absence of kidney disease in all study participants at baseline, the closely supervised, low to moderate-intensity exercise did not result in kidney damage or alterations that exceeded the kidney compensation, thus precluding significant observable variations in urea levels.
To the best of our knowledge, this is the first systematic review and meta-analysis to investigate the association between changes in PA and kidney biomarkers in people without known kidney disease. The studies included underwent rigorous assessment based on strict criteria. We observed low heterogeneity among most of the biomarkers studied. Sensitivity analyses aligned with our primary findings.
It is unlikely that any rise in SCr with PA represents an adverse effect of PA on kidney function, given the widespread benefits of PA on cardiovascular health. It is theoretically plausible that PA reduces glomerular perfusion and, hence, creatinine rises. This effect is seen in people taking both medications inhibiting the renin-angiotensin system46 and sodium-glucose transporter 2 inhibitors.47 This transient rise in SCr is associated with long-term cardiovascular and kidney benefits with these agents. Studies of PA with long durations are required to determine if any change in creatinine with PA is associated with benefits or harm on cardiorenal health.
Although efforts have been made in this study, there are several limitations. First, most studies had a very small sample size, with only a few exceptions. This may, in part, be attributed to a general insufficiency of resources, such as funding and personnel. Second, over 50% of the studies were found to have considerable bias, primarily stemming from high attrition rates, negatively impacting the quality of these studies. Finally, some studies were conducted decades ago, which could introduce potential issues with measurement methods, accuracy and lab standards. This underscores the pressing requirement for updated and standardised research.
In conclusion, by examining the changes in PA among individuals without diagnosed kidney disease, the findings of this study supported the positive association of PA with SCr. However, the association with kidney function specifically could not be confirmed by existing data on other kidney biomarkers. Given the global advocacy for increased PA by governments and medical professionals and the clinical importance of kidney function, further research should be conducted in the general population to investigate the association of changes in PA with kidney function.
Data availability statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors Conceptualisation: PW, QL; Methodology: QL; Data curation: QL, PW, CC-M, PM; Writing—original draft preparation: QL; Writing—review and editing: QL, PW, CC-M, JL, PM; Supervision: PW, CC-M, PM; Critical revision for important intellectual content: PW, CC-M, JL, PM. All authors have read and agreed to the published version of the manuscript. QL is the guarantor.
Funding This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. JL is personally funded by a Wellcome Trust Early Career Award (301005/Z/23/Z).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.