Article Text
Abstract
Introduction Transurethral resection of the prostate (TURP) is the gold standard surgical treatment to lower urinary tract symptoms and benign prostatic obstruction (LUTS/BPO). Although it has been proven to have substantial efficacy in improving functional outcomes, it has shown a high incidence of complications, including transurethral resection syndrome, massive bleeding, urinary incontinence and sexual dysfunction. High-frequency irreversible electroporation (H-FIRE) is a novel non-thermal ablation technique that delivers pulsed high-voltage but low-energy electric current to the cell membrane, thereby leading to cell death. H-FIRE has been reported to be tissue-selective, which leads to fewer side effects. However, no data are available on whether H-FIRE is non-inferior compared with TURP in treating patients with LUTS/BPO regarding safety and efficacy.
Methods and analysis This trial is a prospective, single-centre, randomised controlled, double-blinded and non-inferiority study in which all men with LUTS/BPO are included. This study aims to determine whether the HI-FIRE is non-inferior to TURP for achieving better functional outcomes as measured by the co-primary outcome of the change from baseline in maximal flow rate (Qmax) and the urinary symptoms by questionnaire of International Prostate Symptom Score (IPSS) scoring at 3 months after surgical treatment. The main inclusion criteria are men with prostatic volume range 30 to 100 mL, Qmax<15 mL/s and IPSS>8. A sample size of 118 participants is required, accounting for a 20% loss. All participants will be randomly allocated at a ratio of 1:1 to the H-FIRE arm (n=59) and the TURP arm (n=59). The primary outcome is to assess the change from baseline in Qmax and IPSS scoring at 3 months after surgical treatment.
Ethics and dissemination Ethical approval was obtained from the ethics committee of Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China. The results of the study will be disseminated and published in international peer-reviewed journals.
Trial registration number ClinicalTrials.gov: NCT05306145.
- Prostate disease
- SURGERY
- Adult urology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Prospective, interventional, randomised controlled trial to compare high-frequency irreversible electroporation with the transurethral resection of the prostate in treating lower urinary tract symptoms and benign prostatic obstruction.
Patients’ outcomes will be assessed 1 month and 3 months after surgical procedure.
A rigorous randomised design and blinding method will reduce bias, increasing the results’ reliability.
This study is conducted at a single centre, which may limit the generalisability of the findings.
Introduction
Benign prostatic hyperplasia (BPH) is one of the most common diseases in men over age 50.1 It is often associated with lower urinary tract symptoms (LUTS) and benign prostatic obstruction (BPO), which make a significant impact on quality of life (QOL) and bring a substantial economic burden. Transurethral resection of the prostate (TURP), using the electrode to resect the enlarged prostatic tissue piece by piece, was the preferred surgical treatment for LUTS/BPO in the 1970s and remains the gold standard to date.2 It has been proven to have substantial efficacy in improving functional outcomes like the maximal flow rate (Qmax), the urinary symptoms, which are assessed by the International Prostate Symptom Score (IPSS), and the QOL.3 4 Despite its promising efficacy, TURP is associated with several complications and side effects, such as transurethral resection syndrome or massive bleeding, which may extend hospital stays and increase treatment costs, as well as urinary incontinence or sexual dysfunction.5 Thus, finding and using another minimally invasive treatment are necessary to achieve a similar functional outcome while reducing the limitations.
Irreversible electroporation (IRE) is a novel non-thermal technique for ablation that uses pulsed, high-voltage but low-energy direct electric current to induce nanometer-scale pores in the cell membrane, thereby leading to cell death.6 Due to the independence of thermal processes, IRE seems to be tissue-selective with minor damage to the connective tissue structure, such as blood vessels or nerves, whereas the thermal ablative techniques may cause collateral damage or be limited by the heat-sink effect.7 8 Typical IRE protocols use 80–120 unipolar pulses with a pulse of 50–100 ms, which may evoke muscle contraction during the procedure and then cause the set ablation area position deviation. Another type of IRE technique called high-frequency IRE (H-FIRE) has emerged to overcome this limitation. The H-FIRE protocol applies a set of bipolar pulse bursts, consisting of several individual 0.5–10 μs pulses, aiming to reduce muscle contraction.9
Although H-FIRE and IRE have been used to treat a malignant tumour in most cases, they have promising potential to treat benign diseases like BPH, which is attributed to their efficacy and safety. In our previous multicentre single-arm objective performance criteria trial in treating localised prostate cancer by H-FIRE, we found that in addition to tumour control, it can significantly improve the functional outcome of LUTS/BPO.10 The IPSS increased by 50.0% 6 months after H-FIRE treatment. At the same time, it has not had many complications, especially a very small influence on erectile symptoms and urinary continence. Based on these data and evidence, we hypothesise that H-FIRE might achieve a similar functional outcome but with fewer side effects than TURP.
Hence, we are conducting this single-centre randomised controlled trial (the GIANT trial) to confirm our hypothesis. The primary objective of this trial is to assess whether the H-FIRE is non-inferior to TURP in improving functional outcomes as measured by the Qmax and IPSS in patients with LUST/BPO.
Trial design
GIANT is a prospective, investigator-initiated, single-centre, randomised controlled, double-blinded and non-inferiority study anticipated to enrol 118 patients with symptomatic BPH at Shanghai East Hospital. Participants will be randomised to the H-FIRE group or the TURP group. The primary objective of this study is to determine whether the H-FIRE is non-inferior to the TURP for achieving better functional outcomes.
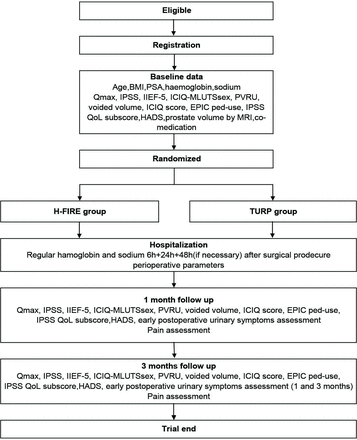
The design overview chart is shown in figure 1, and the details and timeframe of the trial are shown in table 1.
Trial flow chart. BMI, body mass index; EPIC, Expanded Prostate Cancer Index Composite; HADS, Hospital Anxiety and Depression Scale; H-FIRE, high-frequency irreversible electroporation; ICIQ, International Consultation on Incontinence Questionnaire; ICIQ-MLUTSsex, International Consultation on Incontinence Questionnaire Male Sexual Matters Associated with Lower Urinary Tract Symptoms Module; IIEF-5, 5-item version of the International Index of Erectile Function; IPSS, International Prostate Symptom Score; PSA, prostate-specific antigen; Qmax, maximal flow rate; QoL, quality of life; PVRU, postvoid residual urine; TURP, transurethral resection of the prostate.
Participant timeline in the study
Outcomes
The co-primary outcome is to assess the change from baseline in Qmax and urinary symptoms by questionnaire of IPSS11 scoring at 3 months after surgical treatment.
The main secondary outcomes are as follows:
To assess the improvement from baseline in erectile symptoms by the 5-item version of the International Index of Erectile Function (IIEF-5)12 scoring and the International Consultation on Incontinence Questionnaire Male Sexual Matters Associated with Lower Urinary Tract Symptoms Module (ICIQ-MLUTSsex)13 at 3 months after surgical treatment.
To assess the change from baseline in postvoid residual urine volume (PVRU) at 3 months after surgical treatment.
To assess the change from baseline in voided volume at 3 months after surgical treatment.
To assess the change from baseline in urinary incontinence by the International Consultation on Incontinence Questionnaire (ICIQ)14 score and separate Expanded Prostate Cancer Index Composite (EPIC)15 pad-use item at 3 months after surgical treatment.
To assess the change from baseline in QOL by IPSS QoL subscore11 and Hospital Anxiety and Depression Scale (HADS)16 at 3 months after surgical treatment.
To assess the perioperative parameters, including operative time, the postoperative hospital stay, haemoglobin declination, serum sodium declination and catheterisation duration.
To assess the early postoperative urinary symptoms including dysuria, urgency or postmicturition pain.
To assess the change from baseline in pain17 at 3 months after surgical treatment.
To assess the adverse events at every visit. It mainly includes transurethral resection syndrome, blood transfusion, clot retention, retrograde ejaculation, urinary tract infection (UTI), fever and other adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE).
Methods and analysis
Patient population
Patients who fulfil all items of inclusion criteria and without any of the exclusion criteria will be considered qualified to register in this trial. The inclusion criteria include age over 40 years, the prostatic volume range of 30–100 mL, the Qmax<15 mL/s and the IPSS>8. The volunteers will not be recruited if they have a history of prostate cancer or patients suspicious of prostate cancer, neurogenic bladder, metal implants in their body, with long time catheterisation (>2 weeks), or previous history of prostatic or urethral surgery. The details of inclusion and exclusion criteria are shown in box 1.
Patients inclusion and exclusion criteria
Inclusion criteria
Age over 40 years
International Prostate Symptom Score (IPSS)>8
Qmax<15 mL/s
Prostatic volume range of 30 to 100 mL, measured by MRI
Fully understand the clinical trial protocol and sign the informed consent
Exclusion criteria
Have a history of prostate cancer or patients suspicious of prostate cancer
Neurogenic bladder
Metal implants in their body
Previous history of prostatic or urethral surgery
With catheterisation more than 2 weeks
Any other conditions that investigator judges that participations who are not suitable for this trial
Randomisation
Participants who meet the criteria and sign the consent form will be randomly allocated 1:1 to the H-FIRE arm or TURP arm by using block-randomisation. The random sequence will be generated by a computerised database independent of the investigator and will be stratified by age (<70 years and ≥70 years) and prostate volume (<60 mL and ≥60 mL). The randomised number will be revealed after the investigator checks the patient’s informed consent and the inclusion and exclusion criteria. Each participant will have an individual randomisation number, which will be recorded in the case report form (CRF).
All participants will be blinded to the treatment allocation to reduce bias in assessing outcomes, especially of the PROs. The researcher responsible for performing the surgical procedure will not go on to participate in the subsequent assessment, data collection and statistical analysis. The researchers responsible for assessment and statistical analysis will be blinded to the treatment allocation. Blinding will only be broken when the statistical analysis has been completed unless the knowledge of the blinded treatment may influence patient care or safety.
Interventions
Blood and urine samples will be collected before H-FIRE for a routine examination. The questionnaire (ICIQ score, separate EPIC pad-use item and IPSS) will be used to assess the baseline urinary symptoms, as well as IIEF-5 and ICIQ-MLUTSsex, will be used to evaluate the baseline erectile symptoms. Also, the questionnaire of QOL by IPSS QoL subscore and the HADS will be used to assess the QOL. In addition, the Qmax and PVRU will be measured.
Surgery procedure
Both H-FIRE and TURP procedures will be performed by one urologist (HW with experience of more than 10 years, with over 200 H-FIRE cases and over 400 TURP cases). This urologist will be blinded to the patient’s name, the trial number and the initial assessment. Also, he will not participate in the subsequent assessment, data collection and statistical analysis.
H-FIRE strategy
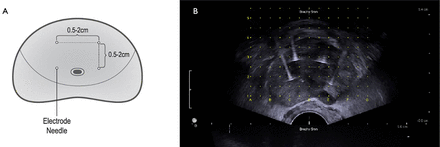
Participants in this arm will receive H-FIRE using the composite steep pulse therapeutic apparatus manufactured by the REMEDINE company. The procedure will be performed in the lithotomy position under general anaesthesia with full-muscle paralysis. Under transrectal ultrasound guidance, the electrode needle will be placed on the target lesion via the perineum by getting through a 5 mm brachytherapy template. Four to six needles will be placed around the middle lobe of the prostate, and the distance between two adjacent needles will vary between 0.5 and 2.0 cm, depending on the size of the prostate. The diagram of electrode needle placement is shown in figure 2. After placing the electrode needle, the apparatus will release the preset pulses. The entire H-FIRE procedure is expected to last 40–60 min. A three-way Foley catheter will be indwelled when the procedure has finished.
Electrode needle placement. (A) Diagram of needle placement. (B) Needle placement under transrectal ultrasound guidance.
TURP strategy
The TURP procedure will be performed with electrodes and a standard tungsten cutting wire loop at 160 W cutting and 80 W coagulating current under general anaesthesia. The resection will begin at the 6 o’clock position of the bladder neck, extending to the verumontanum, and carried down to the surgical capsule, with tissue removal from the gland’s centre zone and transition zone. After the procedure, a three-way Foley catheter will be inserted.
After surgery and follow-up
Irrigation will be applied to all patients, lasting at least 1 day, and will be stopped based on the urine colour. All patients will receive a dose of antibiotic prophylaxis (cephalosporins or quinolone) on the first day after surgery and routinely last 7 days. The duration of antibiotics may be altered or extended depending on particular circumstances. α1-adrenergic receptor antagonists can be used to relieve the symptoms of bladder spasms and will be abandoned after removing the catheter. Other prostatic drugs cannot be used after surgery.
Regular follow-up will begin 1 month after the surgical procedure and continue up to 3 months. The details of each visit are shown in table 1.
Sample size
We will calculate the sample size based on the change in both Qmax and IPSS as co-primary outcomes and ultimately use the maximum of the two calculated sample sizes. Literature showed the minimally clinically important differences were 4 mL/s for Qmax and 2.5–3 points for IPSS.18 19
Sample size calculation based on change in Qmax (larger is better).
Under the non-inferiority hypothesis, a margin of −4 mL/s is set for change in Qmax. It is assumed that changes in Qmax are equal for both treatment groups (H-FIRE and TURP), and the SDs for the two groups are 6.0 mL/s. Patients were randomised to H-FIRE and TURP in a 1:1 ratio. With a target power of 90% and a one-sided type I error rate of 2.5%, the sample size calculation dictates 49 participants per group. Accounting for a 20% withdrawal/loss rate, the total sample size is adjusted to 118 participants.
Sample size calculation based on change in IPSS (smaller is better).
Under the non-inferiority hypothesis, a margin of 3 points is set for change in Qmax. Assuming equal changes in IPSS between the H-FIRE and TURP groups and equal SDs of 4.5 points, patients are randomised in a 1:1 ratio. With the same target power and type I error rate, 49 participants per group are required. Adjusting for the same withdrawal/loss rate, the total sample size is also 118 participants.
The final sample size is determined as a total of 118 participants (59 participants in the H-FIRE group and 59 participants in the TURP group).
Analysis plan
Categorical variables will be described using frequencies and percentages. Continuous variables will be described as mean±SDs for normally distributed variables or as median (the first quartile, the third quartile) for non-normally distributed variables. Between-group comparisons will be evaluated using the Fisher exact test for categorical data, Student’s t-test for normally distributed continuous data and the Wilcoxon rank-sum test for non-normally distributed continuous data.
It is a non-inferiority trial that adopts co-primary endpoints, including changes in Qmax and IPSS from baseline to 3 months. The primary outcomes will be analysed following the intention-to-treat principle as well as the per-protocol principle. The differences in change in Qmax (larger is better) and change in IPSS (smaller is better) between the H-FIRE and TURP arms (H-FIRE minus TURP) will be evaluated with 95% two-sided CIs. The H-FIRE will be described as non-inferior if the lower bound of the 95% CIs of the difference for change in Qmax is greater than −4 mL/s, and the upper bound of the 95% CI of the difference for change in IPSS is less than 3 points.
The secondary outcomes will be analysed following the per-protocol principle. Findings from secondary outcomes will be interpreted as exploratory to avoid potential multiplicity. 95% CIs and P values will not be adjusted for multiple outcomes.
Prespecified subgroup analysis
The subgroup analysis will be performed according to prespecified population characteristics. The characteristics are shown as follows:
Age: <70 years versus ≥70 years.
Body mass index (BMI): <25 versus ≥25.
Prostate volume: <60 mL versus ≥60 mL.
Catheterisation status (before surgery): No catheter versus with catheter.
Bladder stone status (before surgery): No bladder stone versus with bladder stone.
Medical treatment of LUTS (before surgery): No drug treatment versus 5α-reductase inhibitors versus α1-adrenergic receptor antagonists versus combination of both drugs mentioned above.
Missing data
Missing data is a common occurrence in clinical research. All efforts will be made to obtain complete information on baseline characteristics, outcomes and corresponding dates. Reporting missing information is crucial for ensuring the study results’ accuracy, reliability and validity. This allows researchers to accurately analyse the data and draw valid conclusions about the safety and efficacy of the intervention being studied. Initially, the missingness pattern for each co-primary endpoint and secondary endpoint will be reported. Subsequently, model-based multiple imputations by chained equations will impute the missing values for analysing primary endpoints. Ten imputed datasets will be generated if the missing rate is less than 20%. If the rate exceeds 20%, 20 imputed datasets will be generated to ensure that our effect estimates are not unduly influenced by Monte Carlo variability.
Harms and adverse events
All harm relevant or not relevant to the treatment will be recorded according to the modified Clavien classification system and the CTCAE. The assessment will be made by researchers blinded to the intervention group from the beginning of the trial to 24 months after the surgical procedure. The main expected harms or side effects are listed below, and the participants will be informed of the risk for these harms or side effects before registration.
The serious adverse events (SAEs) are defined as any of the following:
Death.
Life-threatening.
Need to hospitalisation or prolonging an existing hospitalisation.
Results in disability or permanent damage.
Congenital deformation or defect.
SAEs will be recorded immediately and then sent to the ethics committee and the GIANT monitoring board within 24 hours. With the occurrence of any adverse events or SAEs, necessary therapy will be taken in time to ensure the safety of the participants.
Data collection
All data will be collected and be entered into a particular database within 48 hours of each study visit.
Case report forms
The collected clinical data will be recorded using CRFs for each enrolled participant. The CRFs should be completed with a black or dark blue pen. Any alterations or corrections on the CRFs need to be signed with the initials and the date at the bottom of the change. All members involved in data collected for GIANT (eg, investigator and research nurse) will be trained to ensure they are familiar with the procedure associated with the CRFs filling.
Data to be collected on screening and inclusion
Basic data collection: name of patient identification, age (year), prostate-specific antigen (ng/mL), prostate volume (mL), Qmax (mL/s), IPSS (scale) and digital rectal examination (DRE) result (normal/abnormal).
Data to be collected during hospitalisation
Data for the participants after the biopsy procedure will be collected as follows:
Basic data collection (before H-FIRE or TURP): BMI, IIEF-5 (scale), ICIQ-MLUTSsex (scale), PVRU (mL), voided volume (mL), ICIQ (scale), EPIC pad-use item (scale), QOL (scale), HADS (scale), pain (visual analogue scale), haemoglobin (g/L), serum sodium (mmol/L) and co-medication.
Data collection about surgical procedure (H-FIRE or TURP): Procedure time (min), weight of resected tissue (g, for TURP).
Data collection after surgical procedure: Irrigation time (day), bladder catheter indwelling time (day, if the catheter had removed during hospitalisation), length of hospital stay (day), transfusion (required/no required), haemoglobin (within 6 hours after surgery, and 24 hours after surgery), serum sodium (within 6 hours after surgery and 24 hours after surgery), pain (visual analogue scale).
Data to be collected during follow-up
Data collection at 1 week: Pain (visual analogue scale).
Data collection at 1 month: Qmax (mL/s), IPSS (scale), IIEF-5 (scale), ICIQ-MLUTSsex (scale), PVRU (mL), voided volume (mL), ICIQ (scale), EPIC pad-use item (scale), QOL (scale), HADS (scale), pain (visual analogue scale) and bladder catheter indwelling time (day, if the catheter had removed after hospitalisation).
Data collection at 3 months: Qmax (mL/s), IPSS (scale), IIEF-5 (scale), ICIQ-MLUTSsex (scale), PVRU (mL), voided volume (mL), ICIQ (scale), EPIC pad-use item (scale), QOL (scale), HADS (scale) and pain (visual analogue scale).
Data retention
CRFs and relative clinical documentation should be kept in a safe place (eg, locked cabinet in a restricted room) and held for at least 10 years after the last publication of the article related to the trial.
Monitoring
Independent team of clinical research associates
An external monitor team of clinical research associates (CRA) will regularly check the data for completeness and quality at least once weekly. They are responsible for being familiar with the trial protocol. The CRA will contact the responsible person demanding the missing data if the CRF needs to be filled out completely and pose queries to the error data, and the validity of the data will only be confirmed if the questions have been resolved. In addition to monitoring the data quality and authenticity, the CRA team will also monitor the researchers and the participants during the whole trial, mainly including:
Monitor the informed consent and the enrolment rate.
Monitor the participants’ retention or withdrawal.
Monitor the harms and adverse events.
Monitor the compliance of participants and investigators with the protocol.
Data safety monitoring board
GIANT will have an independent data safety monitoring board (DSMB) to oversee trial safety.
The DSMB’s role is as follows:
Monitor the trial protocol and informed consent documents.
Monitor the trial’s progress, such as participant recruitment, retention or withdrawal.
Monitor the safety data during the trial (from enrolled to 2 years after surgery).
Monitor the compliance of participants and investigators with the protocol.
Monitor data quality, timeliness and authenticity.
Based on the monitoring, the DSMB can make the recommendations on whether the trial should continue without change, be modified or be terminated.
Ethics and dissemination
Ethical approval was obtained from the ethics committee of Shanghai East Hospital. Informed consent will be obtained from all participants using the supplement. The results of this trial will be disseminated to an international peer-reviewed journal and disseminated for presentation at an international or national academic conference.
Trial status
This RCT was first registered online at ClinicalTrials.gov on 7 March 2022. The study had started on 30 June 2022. Recruitment is anticipated to continue until 1 January 2026, with a 3-month follow-up to be completed in April 2026.
Patient and public involvement
This trial protocol was written without patient or public involvement. The participants were not involved in the contribution of the design, recruitment or conduction of the study. Each participant will be informed of the latest results at follow-up and receive a summary of the main findings at the end of the trial.
Ethics statements
Patient consent for publication
References
Footnotes
B-MH, Z-KS and RC contributed equally.
Contributors BH is the guarantor. Conceptualisation: BH and HW. Data curation: BH, Z-KS, RC and HW. Formal analysis: BH, Z-KS, and RC. Investigation: BH, Z-KS, RC and HW. Supervision: HW. Original draft: BH. Review and editing: Z-KS, RC and HW.
Funding This trial is supported by National Natural Science Foundation of China, youth project (82303612), National Natural Science Foundation of China (82272905), National Natural Science Foundation of China (No. 82373048), Clinical and Basic Research on Prostate Cancer at Dongfang Hospital Affiliated to School of Medicine of Tongji University (No. DFRC2020006), The clinical application of irreversible electroporation ablation in the treatment of metastatic prostate cancer at the Development Center of Shanghai Shenkang Hospital (No.SHDC12023107), Pudong New Area Health Commission Prostate Cancer Special Disease (No. PWZzb2022-04), Pudong New Area Science and Technology Project Pudong New Area Health System Discipline Leader (No. PWRd2020-17).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.