Article Text
Abstract
Background At the European level, several regulatory measures (ie, priority medicines (PRIME) scheme, accelerated assessment, conditional marketing authorisation and authorisation under exceptional circumstances) are in place with the aim to expedite the marketing authorisation process for medicines targeting unmet medical needs (UMNs). However, the potential impact of these measures on subsequent decisions regarding market access at the national level, and ultimately if medicines making use of these supporting measures reach the patient earlier, remains unclear.
Objectives This study seeks to (1) assess the impact of such European regulatory measures on the number of successful applications and time to reimbursement of this group of medicines in the national context of Belgium and (2) evaluate the association between the application of European regulatory measures and Belgian measures (ie, early access pathways and managed entry agreements).
Design A total of 322 medicines granted a European centralised marketing authorisation between 2015 and 2020, excluding generic products/biosimilars, were included in the study. For this set of medicines, data on European and Belgian regulatory and market access measures were extracted from the websites of the responsible European and Belgian authorities and completed with requested information up to December 2022. Regression analysis was used to assess the association between the application of European regulations and Belgian measures. Survival and regression analysis was used to test the impact of such regulatory measures on the time to and rate of reimbursement in Belgium.
Results From the total sample (n=322), 34% (n=108) received a European regulatory measure, and also 34% (n=108) had a Belgian measure applied. Overall, 63% (n=202) of the total sample was submitted for reimbursement in Belgium, and of these, 83% (n=167) were reimbursed at the time of assessment. The median regulatory assessment time at the European level was approximately 14 months, while the median Belgian reimbursement assessment time was approximately 11 months. The study found that regulatory measures did not significantly impact the European or national assessment times or status. A significant reduction in European regulatory assessment time was observed only in the cases of the PRIME scheme (p=0.0087) and accelerated assessment (p<0.0001). The study also indicated a positive association (p=0.0019) between the application of European measures and the application of Belgian measures. However, this significant association was not found for specific measures individually, with the exception of the accelerated assessment (p<0.0001). Medicines undergoing accelerated assessment were more likely to also receive a Belgian measure.
Conclusion This study shows that while European regulatory measures targeting UMNs often trigger corresponding actions in Belgium, this alignment does not necessarily shorten the time from regulatory submission to reimbursement. Lacking submission for reimbursement by pharmaceutical companies appears to be the most frequent reason for absent reimbursement in Belgium. European policy initiatives promoting timely market entry across member states could be crucial for improving patient access.
- Health policy
- PUBLIC HEALTH
- HEALTH ECONOMICS
- Health Services Accessibility
- STATISTICS & RESEARCH METHODS
- THERAPEUTICS
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Health policy
- PUBLIC HEALTH
- HEALTH ECONOMICS
- Health Services Accessibility
- STATISTICS & RESEARCH METHODS
- THERAPEUTICS
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study provides novel insights into the impact of European regulatory measures on Belgian reimbursement timelines and decisions and includes a large sample size, encompassing all product types and disease areas.
The analysis encompasses both the total time from marketing authorisation application to reimbursement and the intermediate time intervals (ie, regulatory assessment timelines and pricing and reimbursement assessment timelines).
Limitations include not considering the cumulative effect of regulatory measures in the statistical analysis, potentially underestimating their overall impact and not including the clock-stop time requested by industry.
Influencing factors such as uncertainties, pivotal trial characteristics, product type, absence of alternative treatments and disease severity were not accounted for, which could affect the results.
Some regulatory measures were applied to a small number of products, limiting the precision of model estimates and statistical power, and the identification of regulatory measures relies on the accuracy of European Medicines Agency publicly available information, which may not always align with the initial assessment report.
Introduction
The European Medicines Agency (EMA) has measures in place to allow earlier, expedited or facilitated centralised marketing authorisation (MA) for medicines that target unmet medical needs (UMNs). These measures include the priority medicines (PRIME) scheme, orphan designation (OD), accelerated assessment (AA), conditional marketing authorisation (CMA) and authorisation under exceptional circumstances (AUEC).1–4 These regulatory measures are in this study referred to as ‘European regulatory measures’. Figure 1 provides more background on these supporting measures, their objectives, eligibility criteria and working mechanisms.
Overview of European regulatory and Belgian measures. EU, European Union; PRIME, priority medicines; R&D, research and development.
These expedited regulatory pathways aim to address the UMNs by allowing medicines to reach the market earlier, often under conditions of heightened uncertainty due to less comprehensive clinical evidence at the time of approval.5–9 However, national health technology assessment (HTA) bodies and payers, such as the Belgian National Institute for Health and Disability Insurance (NIHDI), have their own distinct processes and criteria for determining pricing and reimbursement.10 11 These processes, which assess factors like therapeutic value, cost-effectiveness and budget impact, often introduce additional complexity to the access landscape. In Belgium, national measures that aim to accelerate access include the compassionate use programme (CUP), medical need programme (MNP), and managed entry agreement (MEA).11 Throughout this study, these measures are referred to as ‘Belgian measures’.
While European regulatory measures focus on safety and efficacy mostly versus placebo, and quality, national HTA bodies and payers operate with different objectives. These bodies prioritise safety and efficacy versus alternative treatment options, economic sustainability and budget impact when determining market access and reimbursement. As a result, alignment between the EMA’s regulatory decisions and the reimbursement processes at the national level is often limited. For example, while the EMA may grant accelerated approval for a medicine addressing a UMN, national HTA bodies may still face challenges in justifying reimbursement based on the same evidence, particularly when uncertainties in clinical benefit or economic impact persist. This divergence can lead to delays in patient access despite expedited regulatory approval. Online supplemental material 1 details the European marketing authorisation and Belgian pricing and reimbursement (P&R) procedure.
Supplemental material
As stressed by the European Commission in its pharmaceutical strategy for Europe, a significant source of misalignment between these levels stems from differing definitions and criteria for UMNs, leading to divergent selection criteria for eligible products to apply supporting national measures and facilitate access.9 12–16 The EMA’s supporting regulatory measures are designed to accelerate access to medicines that address critical gaps in treatment, yet the national HTA bodies may use different criteria to assess the same medicines, focusing more on budgetary concerns, cost-effectiveness or clinical evidence thresholds. This lack of alignment in UMN definitions and criteria across regulatory and national HTA frameworks may contribute to delayed patient access in individual countries like Belgium, especially for products granted authorisation with increased regulatory flexibility in the form of conditional authorisation.9 12–14
Furthermore, medicines approved through these European regulatory pathways rely on incomplete or immature clinical data. While this approach facilitates earlier market entry, it may result in longer delays in national reimbursement decisions or even negative outcomes, as HTA bodies typically require robust evidence of added benefit to justify reimbursement.15 17 The risk here is that medicines approved conditionally or with reduced evidence thresholds at the European level may struggle to meet the stricter national criteria, thereby delaying patient access. It is currently unclear whether medicines benefiting from the application of these supporting European regulatory measures will ultimately reach patients sooner.
Previous studies have suggested that the application of European regulatory measures does not consistently lead to a shorter time to market entry or an increased likelihood of reimbursement in specific countries.5 12 18 However, these earlier studies are often limited in time, focused on a certain country (excluding Belgium), and/or a specific disease area, predominantly oncology. The objective of this study is to (1) evaluate the association between the application of European regulatory measures and Belgian measures for centrally authorised medicines that addressing UMNs, and (2) assess the impact of such European regulatory measures on the number of successful applications and time to reimbursement of this group of medicines in Belgium.
Methods
Selection of medicines
All products that were centrally authorised by the European Commission between 2015 and 2020 were included for the analysis. The generic medicines and biosimilars but also indication extensions were excluded. Furthermore, products that were either withdrawn or refused regulatory authorisation by the European Commission after a positive opinion of the EMA’s Committee for Medicinal Products for Human Use (CHMP) were also excluded from the analysis.
Data sources and data extraction
All European regulatory and Belgian measures that had UMN as either a direct or indirect eligibility criterion were included in this analysis (online supplemental material 2). European regulatory measures include: PRIME scheme, AA, CMA, AUEC and Belgian measures include: CUP, MNP and MEA. Related extraction parameters per included medicine are the European and national regulatory and market access measures (eg, AA, CMA, MEA) and relevant time points as presented in figure 2. Information on regulatory measures taken within the assessment and authorisation process (ie, PRIME, OD, AA, CMA, AUEC), date of applicants’ submission for centralised MA (t0) and the date the MA was granted (t1) was extracted from the European Public Assessment Report (EPAR) displayed on EMA’s website.
Visual representation of the studied time points of regulatory and pricing and reimbursement activities and time differences. Timepoints were extracted as dates, and time differences in months. Figure inspired by conceptualisation in publication by Ferrario.18 EMA, European Medicines Agency; MA, marketing authorisation; P&R, pricing and reimbursement.
Decision dates on (pricing and reimbursement) P&R (t3) were extracted per medicine from the website of the Belgian National Institute for Health and Disability insurance (NIHDI).19 The regulatory and P&R assessment times were analysed including clock-stops requested and obtained by the applicants. When P&R dossiers were resubmitted for the same medicines, the date of first submission were used for the assessment. Information relating to the date of applicants’ submission for P&R (t2), managed entry agreements (MEA), and early access programmes (CUP, MNP) were provided by the national responsible authority, respectively the Belgian payer NIHDI, and Belgian competent authority Federal Agency for Medicine and Health Products (FAMHP) on request. Data are up to date up to and including December 2022 with extraction performed at the end of December 2022, encompassing all European and Belgian regulatory and P&R decisions made up to that point. However, the analysis only includes medicinal products that were centrally authorised between 2015 and 2020.
Descriptive analysis
A descriptive analysis was conducted to provide an overview of the characteristics of the medicinal products included in this study. Key characteristics analysed included the year of initial MA, anatomical therapeutic chemical (ATC) classification and the application of European regulatory and Belgian national measures. The WHO explains the ATC system as ‘a system that divides drugs into different groups according to the organ or system on which they act and their chemical, pharmacological and therapeutic properties’.20 In addition, the descriptive analysis tracked actions taken to obtain reimbursement in Belgium for each product associated with the European regulatory measures. This analysis involved identifying whether reimbursement dossiers were submitted, and assessing the outcomes of the reimbursement process (ie, positive decision, negative decision, or dossier closure at the request of the marketing authorisation holder).
Statistical analysis
All conducted statistical analyses are regression models with regulatory measures as independent variables and the type of regression model (logistic, linear or Cox) depending on the type of outcome. Outcomes can include (1) a positive outcome, where a positive reimbursement decision was obtained, and (2) a negative outcome, where no reimbursement was granted at the time of analysis due to a negative reimbursement decision, the reimbursement application being closed by the MA holder, or no outcome (censored), where the medicine has not yet been submitted for reimbursement. Note however that for all medicines in the negative category the time until reimbursement was considered as being censored since all these medicines may still achieve reimbursement in the future.
European regulatory measures (ie, PRIME, OD, AA, CMA, AUEC) are not mutually exclusive and often combinations are present (eg, a medicine can obtain an AA in combination with a CMA), some of the European regulatory measure groups only containing a few medicines. Therefore, for each possible measure, a separate model was fitted comparing the group of medicines with that measure with the group of medicines with no measure at all. Hence, each of these models were fitted on a subset of the data. In each of these separate models, a correction was made for confounding factors year of MA (continuous) and ATC code (categorical with eight levels). In an additional model, fitted on all included medicines, an independent variable ‘any measure’ was defined, covering the application of any regulatory measure, without the distinction for the kind of measure. All analyses were performed using the SAS software (V.9.4) and p values<0.05 were considered significant.
Impact of European regulatory measures on the assessment time to and number of centrally authorised medicines receiving reimbursement in Belgium
In the first part of the analysis, the association between European regulatory measures and (1) the overall assessment time (∆tOverall), (2) the regulatory assessment time (∆tMA process) and (3) the P&R assessment time (∆tP&R process) were assessed.
Since some products did not yet receive reimbursement by December 2022, survival analyses (Cox regression) were used to evaluate the relation between regulatory measures and the time from MA application to reimbursement. Products that did not (yet) obtain reimbursement in Belgium were censored. The ‘survival’ time was defined as the interval between the MA application and the date of reimbursement (∆tOverall). The proportional hazards assumption was assessed via the supremum test.21 The same approach was used for the relations with (∆tP&R) but restricted to medicines that were submitted for reimbursement in Belgium at the time of analysis.
Since all medicines included in the analysis obtained centralised MA, multivariable linear regression models were used for the time between MA application and centralised MA (∆tMA process). The distribution of the model residuals was inspected and the normality assumption was quantified with the Shapiro-Wilk statistic.
Association between European regulatory measures and the adoption of Belgian measures
The second part of the analysis consisted of a multivariable logistic regression model with the application of Belgian measures (yes/no) as dependent variables. The rationale for grouping national measures lies in their shared underlying intent: both are applied to medicines perceived as addressing significant patient needs, aligning with the rationale behind many European regulatory measures. This analysis was limited to medicines that were submitted for reimbursement in Belgium, ensuring that all medicines were equally eligible for any of the considered Belgian measures. The Hosmer-Lemeshow goodness-of-fit test as well as the Stukel test were used to verify the goodness-of-fit.22
Patient and public involvement
Patients and the public were not involved in the design, or conduct of this research.
Results
Between 1 January 2015 and 31 December 2020, 513 human medicinal products obtained centralised MA by the European Commission, of which 52 were withdrawn/revoked. From the remaining 461 products the biosimilars (n=40) and generic (n=99) products were excluded resulting in a final sample of 322 medicinal products. Table 1 provides an overview of the characteristics of included medicinal products.
Characteristics of medicinal products authorised by the EMA between 2015 and 2020
Impact of European regulatory measures on the reimbursement status in Belgium and assessment times
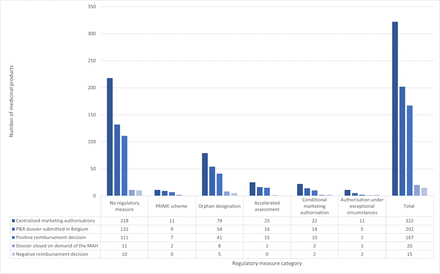
Figure 3 provides an overview of the actions undertaken to obtain reimbursement in Belgium for every European regulatory measure category, including the submission of the reimbursement dossier, the result of the reimbursement procedure (ie, positive reimbursement decision, dossier closed on demand of the MA holder, or negative reimbursement decision). Within the sample (n=322), after 5 years since the MA application, 167 of medicines obtained reimbursement in Belgium. After 5 years, the highest reimbursement rates were associated (but not statistically significant) with medicines included in a PRIME scheme or those using AA. From the total number of included products (n=322), 202 (63%) were submitted for reimbursement in Belgium by the MA holder, of which 167 (83%) were reimbursed at the time of analysis. Of the remaining submitted medicines that were not reimbursed in Belgium (n=35, 17%), 20 (57%) MA holders closed their dossier after submission, and 15 (43%) received a negative reimbursement decision. The distribution of the sample across ATC codes and authorisation years is detailed in online supplemental material 3, which includes descriptive data presented in two graphs.
Actions taken in the process to obtain reimbursement in Belgium for every European regulatory measure category (ie, no regulatory measure, PRIME scheme, orphan designation, accelerated assessment, conditional marketing authorisation, authorisation under exceptional circumstances). BE, Belgium; MAH, marketing authorisation holder; PRIME, priority medicines; P&R, pricing and reimbursement.
Figure 4 presents the median assessment time per European regulatory measure category for (1) the regulatory assessment, (2) the P&R assessment and (3) the time between regulatory submission and reimbursement decision. It also includes the association between the respective regulatory measures and (time to) reimbursement. The only statistically significant association was found for the application of PRIME (p=0.0087) and AA (p<0.0001) on the European regulatory assessment time. The overall median time from MA application to centralised European MA for the entire dataset (n=322) was 14.0 months, whereas for AA it was only 8.3 months, followed by PRIME at 11.2 months. In contrast, other European regulatory measures (CMA, AUEC, OD) did not result in a statistically significant change in European regulatory assessment time or Belgian P&R assessment time.
Median assessment time per European regulatory measure category for (1) the European regulatory assessment, (2) the Belgian P&R assessment and (3) the time between European regulatory submission and Belgian reimbursement decision. ND, not defined; MAH, marketing authorisation holder; P&R, pricing & reimbursement. *This analysis is applied to the fraction of the medicines that had been submitted for reimbursement at the time of the analysis. #proportional hazards assumption violated.
Notably, medicines granted an AA exhibited the shortest median time to reimbursement, with a median duration of 10.1 months from dossier submission, compared with a median of 10.7 months for the overall sample. However, this difference was not statistically significant (p=0.5127). Moreover, the P&R assessment process time was even longer for medicines included in the PRIME scheme, with a median of 15 months. Similarly, medicines that obtained any other European regulatory measure showed longer P&R assessment times but these differences were not statistically significant, suggesting no significant reduction in P&R assessment time for medicines subject to these regulatory measures.
Finally, the lowest median times between MA application and reimbursement were observed for products included in PRIME (37.8 months) and AA (44.5 months). But also here there was no statistically significant association between the application of European regulatory measures and the overall time between MA application and Belgian reimbursement. More detailed results and Kaplan-Meier graphs can be found in online supplemental material 4.
Association between European regulatory measures and the adoption of Belgian measures
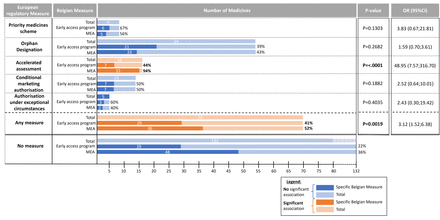
Figure 5 presents the fraction of medicinal products included in a Belgian measure and the association of the European regulatory measure and the subsequent application of Belgian measures. Of the 202 medicinal products that were submitted for reimbursement in Belgium, 58 (29%) were subject to an early access programme (CUP and/or MNP) in Belgium. For these 58 products, the highest inclusion rates for early access programmes were found for products that were part of a PRIME scheme, for which six out of nine medicines submitted for reimbursement were included in an early access programme (67%), followed by the AUEC (60%), CMA (50%). Moreover, 22% of products for which none of the regulatory measures at the European level applied were included in an early access programme in Belgium. Furthermore, 48 (36%) products were included in an MEA. Products with the highest MEA inclusion rates, were products that received AA (94%), followed by products included in the PRIME scheme (56%). Overall the inclusion rates of products that received a European regulatory measure were higher (52%) than for products that did not get a European regulatory measure (36%).
Fraction of medicinal products included in a Belgian measure. MEA, managed entry agreement. Belgian early access programs include compassionate use program and/or medical need program.
When products obtained any regulatory measure by the EMA and were submitted for P&R assessment in Belgium, they were more likely to obtain a Belgian measure (p=0.0019). When testing this for respective European regulatory measures separately, only medicines that obtained an AA were more likely to also obtain a Belgian measure at the Belgian level (p<0.0001). For all other European regulatory measures, there were no significant relation with the subsequent application of Belgian measures.
Discussion
This study assessed medicines, centrally authorised by the EMA between 2015 and 2020, where supporting measures at both the European (eg, PRIME scheme, OD, AA, CMA, AUEC) and national levels (eg, CUP, MNP, MEA) aimed to facilitate the authorisation and reimbursement of medicines, relying on the UMN concept as an eligibility criterion. However, it is known that the lack of a universally accepted legal definition of UMN created uncertainties in its application.15 16 This study examines the impact of these regulatory measures on the duration and outcomes of the European and Belgian processes and explores the alignment between European and Belgian supporting measures.
This study demonstrates that while medicines benefiting from European regulatory measures aimed at addressing UMNs are more likely to prompt corresponding actions at the Belgian level, this alignment does not necessarily lead to a shorter overall timeline from regulatory submission to final reimbursement and patient access. At the European level, only AA and PRIME schemes are associated with shorter regulatory review times, whereas CMA, AUEC and OD do not show similar effects. Importantly, this reduction in regulatory timelines at the European level does not translate into faster reimbursement decisions in Belgium or a shorter overall timeline. Most medicines that were not reimbursed at the time of analysis could be explained by lacking submission of the reimbursement dossier in Belgium.
The shorter assessment time for AA and PRIME medicines is not surprising, as with AA the number of active days (excluding clock-stops) of regulatory assessment is reduced from 210 to 150 days and PRIME medicines are automatically also eligible for AA.4 23 This finding was confirmed in the literature, and in a cohort of oncology medicines also those products that were reviewed under AA had shorter assessment times.24 Also, one earlier study found shorter regulatory assessment times for products included in PRIME.25 In contrast to the findings of this present study, another study assessing the regulatory assessment time of oncology medicines in Europe found a significantly longer assessment time for medicines that obtained a CMA.7 Nevertheless, conditionally authorised medicines may not have a shorter regulatory assessment time, but they can be approved with less comprehensive clinical data if the immediate benefits outweigh the risks of needing more data.7 26 27 Medicines authorised under UEC or with OD were not evaluated in a shorter timeframe, which can likely be attributed to their complex nature. Both orphan and UEC medicines often face significant challenges in generating robust clinical evidence due to the rarity of the conditions they treat or the difficulty in conducting traditional trials. Despite these challenges, both regulatory measures provide crucial pathways for these medicines to gain authorisation and become accessible to patients. Notably, the EMA provides tailored scientific advice and early guidance for medicines with OD, as well as those in the PRIME scheme, which can help streamline the regulatory process even for complex products. This scientific support likely mitigates potential delays, allowing these medicines to proceed through regulatory review without significant extension of timelines, despite the complexities involved. Without such frameworks, the availability of treatments for conditions affecting small or hard-to-study patient populations would be severely limited.
When the EMA grants authorisation, a certain level of uncertainty is always present, though some authorisation types, such as CMA and authorisation UEC, allow for higher degrees of uncertainty. This uncertainty is balanced against the potential benefit the medicine offers to patients and its contribution to addressing a UMN. Only when a medicine is expected to provide substantial benefit are these more flexible approval pathways granted. It is important to note that Brinkhuis et al found more frequent reports of negative or non-quantifiable added benefits for medicines with CMA compared with those with standard marketing authorisation.17 Similarly, Hwang et al observed no statistically significant correlation between high clinical benefit and shorter overall review times.28 Despite these uncertainties, reimbursement is crucial to ensure that these often high-cost medicines are accessible to patients. A recent study investigated the types of uncertainties remaining at the market access level and provided guidance for managing them within HTA processes, proposing mitigation strategies.29
Given the differing objectives of the EMA and national HTA bodies or payers, the acceleration seen at the European regulatory level does not seem to translate into faster timelines at the national level. In the present study, of the included medicines, 52% were reimbursed in Belgium at the time of analysis, closely matching the 51% availability indicated by the EFPIA W.A.I.T. (Waiting to Access Innovative Therapies) indicator for the period from 2018 to 2021.30 Nevertheless, the present study found that the main reason for non-reimbursement, accounting for 77% of non-reimbursed medicines, was the lack of submission by the MA holder in Belgium. To address this issue, the European Commission has proposed additional regulatory data protection in the revised pharmaceutical legislation for Europe, provided the product is made available in all member states.31 This proposed change could incentivise MA holders to submit reimbursement dossiers promptly after obtaining an MA by the EMA. Launch delay, defined by Büssgen et al as ‘the difference between the first international launch date and the corresponding national launch date’, tends to be negatively correlated with expected price and market size.32 Although Büssgen and Stargardt reported that launch delays decreased between 2000 and 2017 for the included countries, Belgium’s decrease appears slower compared with peer countries, placing Belgium 19th out of 30 European Union (EU) countries studied during the period of 2015—2017.32 Delays in the launch of medicines in Belgium can be attributed to various factors, including the relatively small market size, the perceived likelihood of reimbursement success, and the size of the pharmaceutical company.33 Additionally, Belgium’s recent decision to publish the public parts of its MEAs may further discourage MAHs from submitting in the country.34 35 Although the confidential nature of MEA pricing is maintained to prevent international reference pricing from lowering prices in other countries, this secrecy may inadvertently contribute to delayed launches in Belgium. Moreover, while changes in P&R regulations are generally found to cause delays in the adoption of pharmaceuticals, the specific impact of these recent regulatory adjustments in Belgium remains to be observed.33
None of the assessed European regulatory measures were found to have a significant impact on the Belgian P&R assessment time, meaning that the application of a PRIME scheme for instance is not associated with faster Belgian reimbursement. However, other scientific studies present inconsistent findings regarding the impact of European regulatory measures on P&R assessment times in other countries, with some results even contradicting each other. One study, for instance, conducted in France, the Netherlands, Portugal, England, Poland and Scotland, found similar variability in P&R decision-making between conditional and standard centrally approved oncology products.12 Another study, including various EU market access authorities (ie, HAS (France), SMC (Scotland), and CADTH (Canada)), suggests that conditionally approved medicines face increased barriers relative to standard authorised medicines, resulting in a prolonged time to reimbursement due to lower levels of clinical evidence and heightened clinical and economic uncertainties.5
While both AA and the PRIME scheme show a significant positive impact at the European regulatory level in terms of reduced assessment time and a significant association between the application of European regulatory measures and Belgian measures, this does not seem to translate into an overall reduction in the time to reimbursement. The results of the present study indicate that the reduced MA assessment time does not compensate for the time between the moment of MA and submission of the P&R dossier and the time it takes to perform a P&R assessment. These timelines depend on decisions made by MA holders and the P&R assessment timelines. Consequently, efforts undertaken at the European level do not appear to directly lead to earlier patient access in Belgium per se. This highlights an important opportunity for increased dialogue and coordination between regulatory evaluation and reimbursement processes on the one hand and a need for timely submission for reimbursement by the MAH on the other hand. The latter is included in the proposal for the revision of the pharmaceutical legislation for Europe as previously mentioned.31
As the UMN concept is one of the critical elements used in the eligibility decision for supporting European regulatory and national measures with the aim of expediting patient access, more alignment between the European and national levels could potentially increase harmonisation and result in expedited patient access to those crucial medicines in practice.15 In 2023, the European Commission adopted a proposal for a new pharmaceutical legislation including a new definition of UMN with the intention of harmonising its implementation.31 36 If all stakeholders, responsible for decisions regarding authorisation and market access of medicines, would agree on a unified, adjusted definition and apply it in their practices concerning the application of supporting regulatory measures and reimbursement decisions, this policy evolvement could potentially contribute to more alignment between/at the European and national level.
In parallel with the reform of the pharmaceutical legislation, Belgian reimbursement procedures were revised to create alignment with European reforms, and new policy proposals are summarised in a roadmap based on multi-stakeholder input.34 35 Within this revision, a key proposal linked to UMN involves its systematic integration into the reimbursement process for novel medicines, more specifically within the decision-making process regarding added value.34 35 In addition, the European PRIME measure is considered a starting point for a suggested new early and fast access procedure to speed up reimbursement of innovative medicines in Belgium.34 35 These efforts aim to better align national with European measures for access to innovative medicines.
Enhancing transparency in European and national decision-making
Currently, there are several digital platforms or databases available to create transparency on the access to medicines in specific geographical regions. At the European level, EFPIA has developed an EFPIA W.A.I.T. Indicator, which aims to assess market access timelines for centrally approved medicines across Europe.30 According to this W.A.I.T. indicator, the rate of availability of medicines in Belgium (between 2017 and 2020) was 54%, similar to the 52% found in this study.30 Nevertheless, the EFPIA W.A.I.T. indicator does not take into account whether a reimbursement dossier was submitted by the MA holder, which is crucial information to be able to estimate the performance of a country. The current study found that the actual reimbursement rate in Belgium after submission of the P&R dossier is 83%. Apart from the European W.A.I.T. indicator, there also exist national tracking systems, such as in the Netherlands governed by the Central Health Professions Centre (CIBG), which includes more detailed information on the specific stage of the reimbursement process a certain medicine is currently situated in. In addition, it also contains information relating to other available avenues, including availability via early access programmes, all specifically within the context of the Netherlands.37 Furthermore, in 2023, the WHO launched the so-called novel medicines platform, where one aim is to create an access dashboard, including access data for the 53 countries in the European WHO region.38 This platform could already form a basis for more transparency on access to medicines and its barriers in the European region.
Strengths, limitations and future research
Strengths
To our knowledge, this is the first study that examines the association between European regulatory measures and downstream national measures, offering novel insights into their impact on Belgian timelines. Moreover, it exhibits a strength in its large sample size of included medicines. In contrast to other studies that narrow their focus to specific medicinal product types (small molecule or biotechnological), disease areas (eg, oncology) or regulatory measures, this investigation adopts a comprehensive approach, encompassing all product types and disease areas in a certain time period (medicines centrally approved between 2015 and 2020).5 7 24 25 39 Of note is the comprehensive examination not only of the overall assessment time from MAA to reimbursement but also the consideration of intermediate time intervals, thereby providing more granularity compared with similar assessments such as the EFPIA W.A.I.T indicator.30 Lastly, the study comes at an important moment in pharmaceutical policy reform and may be informative to future policy proposals on regulatory and market access measures to facilitate or expedite decisions.
Limitations
There are four limitations to consider when interpreting the findings of this study. First, none of the statistical analyses performed took the cumulative effect of regulatory measures into account. Instead, the measures were considered separately, which could have resulted in an underestimation of their overall impact. Second, besides ATC code and year of MA, other influencing factors such as uncertainties, pivotal trial characteristics, type of the product (small molecule or biotechnological), the absence of alternative treatments, firm characteristics and disease severity that could influence the assessment were not corrected for in this study, which could have influenced the results.5 24 33 39 40 Third, it is important to note that some regulatory measures were only applied to a small number of products, which limits the precision of model estimates and statistical power. Caution must be exercised when interpreting the individual effect sizes of these smaller groups in the multivariate model. Additionally, the statistical power of the model may not have been sufficient to detect all relevant effects due to the limited number of products to which some regulatory measures were applied. Therefore, the findings of this study should be interpreted with this limitation in mind. Finally, the identification of regulatory measures such as AA, CMA and AUEC, relies on information extracted from the EMA website. It is noteworthy that, in certain cases, this data may not align with the information presented in the initial assessment reports, particularly when instances occur wherein a CMA transitions to a full MA.
Future research
While the observed differences in reimbursement timelines suggest potential trends across various regulatory measures, the limitations in sample size, variability, and the absence of additional statistical metrics advise caution in interpreting these results. Further research with larger datasets and more robust statistical analysis will be essential to confirm these preliminary observations and to better understand the impact of these regulatory pathways on reimbursement timelines. Additionally, this study did not specifically assess the impact of clock-stops requested by the MA holder. Clock-stops, which are included in our analysis as an integral part of both regulatory and P&R assessment periods, allow time for additional data submission or clarification and may substantially extend the perceived timeline to approval and reimbursement. Future studies could explore the influence of clock-stops in greater detail to provide further insights into these extended timelines. This study acknowledges the complexity of the medicinal product development journey. Factors such as scientific advice and the PRIME scheme can impact (pre-)clinical development time, potentially expediting overall accessibility for patients. Therefore, the specific impact of these measures on development time must still be assessed in the future. Further, it is crucial to recognise that national reimbursement does not guarantee immediate patient access. Steps post-reimbursement, like drug launch, prescription, and medicine availability, are not covered in this study and require separate examination. Furthermore, more in-depth studies are needed to identify the most relevant regulatory and/or market access indicators and develop instruments, such as databases or web-based tools, to create transparency on the access to medicines across the European member states, or even broader.
Conclusion
This study demonstrates that while medicines benefiting from European regulatory measures targeting UMNs, are more likely to trigger corresponding actions at the Belgian level, this alignment does not necessarily result in a shorter overall timeline from regulatory submission to final reimbursement and patient access. For treatments genuinely addressing critical health needs, increased coordination between European and national levels could be advantageous. However, lacking submission for reimbursement by pharmaceutical companies appears to be the most frequent reason for absent reimbursement in Belgium. Therefore, European policy initiatives that encourage timely market entry across all member states may have the greatest impact on improving patient access to new treatments, particularly in countries like Belgium.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the organisations with which the authors are affiliated. We thank Francesco Pignatti of the EMA for his invaluable contributions. His critical reflections on the methods, as well as his expertise in visualising and interpreting the results, significantly enriched the quality of this research. We would also like to express our appreciation to Inneke Van De Vijver, also from RIZIV/INAMI, and Joke D'Hoker of the FAMPS, for their support to provide data and share insightful perspectives. Thanks to Anais Brouckaert for the input in the educational chapter.
References
Footnotes
Contributors The study design was collaboratively developed by all coauthors (ZC, SF, JD, LB and IH). National data were provided by the responsible organisation, facilitated by Inneke Van De Vijver from RIZIV/INAMI, and Joke D’Hoker from FAMPS. Issues related to data accuracy were addressed by ZC and JD in collaboration with agency contacts. ZC conducted data extraction and initial analysis, with statistical validation performed by SF. ZC and SF resolved statistical issues. ZC led the writing and visualisation of the data, under the supervision and validation of SF, LB, JD and IH. Francesco Pignatti provided written input and suggestions regarding manuscript structure. IH is the guarantor. Artificial intelligence assistance, specifically through the OpenAI ChatGPT model, was used to refine text in this manuscript. The final text was still validated and adapted and hence an original product of the authors.
Funding Funding for this research was provided by IMI CARE. The CARE project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 101005077. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and BILL & MELINDA GATES FOUNDATION, GLOBAL HEALTH DRUG DISCOVERY INSTITUTE, UNIVERSITY OF DUNDEE. The content of this publication only reflects the author’s view and the JU is not responsible for any use that may be made of the information it contains. This study was supported by the KU Leuven. Researcher ZC is SB PhD fellow at FWO - 1S52125N.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.