Article Text
Abstract
Introduction As one means to avoid waste in research investment, involving patients as full partners in research has become increasingly frequent. There is clearly a low level of investment in palliative care research. Following the guidance from the James Lind Alliance and the UK public consultation (‘Palliative and End of Life Care Research Priorities Project’), we developed a 3-step protocol aimed at prioritising 10 unanswered questions in palliative care (PC) research in France, from the viewpoint of patients, volunteers, healthcare professionals and family caregivers.
Methods and analysis To identify unanswered questions in PC (stage 1), an unstructured questionnaire will be used. This questionnaire will be tested on patients and healthcare professionals and modified, if necessary, before being made available online for a period of 6 months. A multidisciplinary steering committee including board-certified PC physicians, methodologists, nurses, a sociologist, an anthropologist and an information specialist will analyse the data collected in order to delete duplicate questions, do a thematic and population classification of the responses, modify questions using the PICO (patient problem, intervention, comparison and outcome) format and perform a literature review on each question to identify any relevant systematic review.
Ethics and dissemination We expect the results to have wide-ranging benefits, for example, by prompting investment in the 10 prioritised research questions. There are also potential benefits for patients and caregivers, by including them as partners in future research. Regarding the current bill being examined by the government planning to legalise euthanasia and assisted suicide in France, this study will provide new insights into how patients and caregivers are prioritising those themes. The major benefit of this study is to involve patients and family caregivers as partners in PC research. They will be consulted and their choices will be valuable resources and may prompt researchers to focus on different topics. In view of the limited funding available, PC research needs to prioritise major issues and raise its visibility.
The second stage of the study is the first-round prioritisation using a fixed format questionnaire, which will last 4 months. The third stage will consist of reaching a consensus regarding the top 10 unanswered questions in PC research, using the nominal group technique. A secondary objective during this third step is to study the reasons for the prioritisation.
- Adult palliative care
- Patient Participation
- Family
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A mixed method to identify and prioritise 10 unanswered questions.
Including patients and family caregivers.
Multidisciplinary steering group: doctors, nurses, an information specialist, an anthropologist, a sociologist and methodologists.
The need to have two different enrolment strategies.
Introduction
Investment in biomedical research amounted to more than US$100 billion in 2022.1–4 Unfortunately, much investment in research is wasted.5–7 Unclear or inappropriate research questions, ambiguous or useless protocols, major biases and difficulties in publishing relevant work are just some of the possible explanations for wasted research funding. A review highlighted that only 53% of studies have been published 9 years afterwards,6 revealing a major gap between investment and results.
Non-communicable diseases, such as cancer or neurological diseases, garner very high levels of research funding, as do high-income countries.8 In palliative care (PC), there is clearly a very low level of investment.9 10 Indeed, investment in PC research accounts for 0.3% of the 700 million GBP invested in research overall in the UK in 2020.9 Furthermore, PC research in patients other than those with cancer, receives even fewer financial resources.9 Yet, research in PC has impacted practice and contributed to improving patients' quality of life.11–13 Nevertheless, further research is needed to quantify and describe this impact,14 15 and additional resources are needed for this purpose. Given the limited resources invested in PC research, it is essential to focus on topics that are most relevant to patients, healthcare providers and family caregivers.9 16
In this regard, it has been shown that surgical and medical treatments are not always the priority for patients. In the literature, gaps between the priorities of researchers and patients have been highlighted. For example, only 9% of patients with knee arthrosis would prioritise treatment research, whereas research into treatments for the disease accounted for 80% of clinical trials.17 18
For this reason, involving patients as partners in research is becoming increasingly frequent. The challenge is to identify the best ways of involving patients in research.19 Finding a consensus on research priorities between patients and researchers is one of the key purposes of patient involvement.20 The inclusion of healthcare professionals and family caregivers is also important.9 16 In the UK, under the auspices of the end-of-life charity ‘Marie Curie’, a public consultation (the Palliative and End Of Life Care Priority Setting Partnership initiative) was organised, including patients, volunteers, health and family caregivers, with a view to prioritise 10 key questions in the area of PC. The top 10 unanswered questions related to access to PC services throughout the UK and access to PC during out-of-working hours; to the information and education of carers and families, the training of healthcare professionals, the impact of advance care planning, how to evaluate and deliver PC to the non-cancer patients in palliative care, how to evaluate and treat pain and discomfort, how to provide care at home, how to pursue continuity for patients in terms of staff and what core PC services should be provided.21–27
In line with the James Lind Alliance guidance21–28 and the UK public consultation,29 we designed a 3-step protocol that aims to prioritise 10 unanswered questions in PC research in France, from the viewpoint of patients, volunteers, healthcare professionals and family caregivers.
Methods and analysis
Definitions
A PC patient is defined as a person suffering from a life-threatening illness and for whom the goal of care is to improve quality of life, by alleviating disease-related symptoms and providing emotional, social and spiritual support.30
In this study, the term ‘healthcare professional’ encompasses doctors, social workers, nurses, nurses’ aides, home care assistants, physiotherapists and psychologists.
A family caregiver31 is a person who tends to the needs of a member of their family (either their family of origin or their family of choice); this includes (but is not limited to) helping with coordination, purchase, preparation or administration of medication, assisting with personal hygiene and toileting, managing meals or finances, nursing or any other form of help provided in day-to-day life.
French health regulations32 define a volunteer, not as a healthcare professional but as a member of a PC team who accompanies and provides psychological and/or social support to the patient and their family, thereby contributing to the quality of their palliative management.
The term ‘unanswered question’ can refer to any topic and any field of research. An unanswered question is a question for which no formal and unequivocal response can be found in the medical literature.
Inclusion and non-inclusion criteria
Each respondent must provide their expertise or the features/circumstances that qualify them to respond (tables 1 and 2).
Inclusion criteria for the third step
Non-inclusion criteria for the third step
For the first and second stages of the study, all patients in PC and family caregivers are considered experts and are therefore eligible for inclusion. Inclusion criteria for PC healthcare professionals and volunteers are: (1) certified university qualification in PC; or (2) more than 2 years experience in a PC team. For all participants, the following inclusion criteria also apply: age >18 years, and the ability to read and speak French.
Inclusion and non-inclusion criteria for the third stage of this study are detailed in tables 1 and 2, respectively.
Objectives and study design
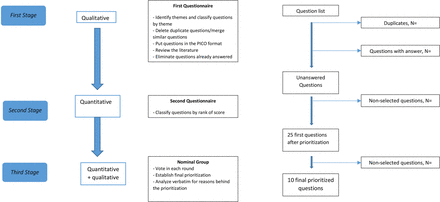
This study’s objectives are to determine and then prioritise the top 10 unanswered questions in PC research in France (figure 1).
Flow chart. PICO, patient problem, intervention, comparison and outcome.
The first step of this study has already been completed. The second step is currently in progress. The start date was 18 January 2022 and the planned end is March 2025. The first stage aimed to identify unanswered questions in PC research. This was done using an unstructured questionnaire (online supplemental file 1). The questionnaire was developed using the guidance from the James Lind Alliance and the UK public consultation ‘Palliative and End of Life Care Research Priorities Project’.21–23 25 29 The questionnaire was tested on a subset of patients and healthcare professionals and modified, before being made available online for 6 months, from 18 January 2022 to 13 July 2022. Following the test, the steering committee subdivided the initial single question into two separate questions, the first asking respondents to cite themes of interest, and the second, asking respondents to cite questions that needed to be answered in each of those themes. The survey was prepared using Limesurvey software. To ensure maximum outreach among patients, a paper version was also available on request, as well as the possibility to complete the survey over the phone. Flyers and posters with the URL link and QR code to reach the questionnaire were made available. The questionnaire was distributed by professional societies of PC in France (namely, the Société Française d’Accompagnement et de Soins Palliatifs (SFAP)) and by a French PC research platform (Plateforme Nationale sur la Fin de Vie), as well as by various groups and associations for patients and caregivers. All healthcare professionals and volunteers who are subscribed to either organisation were contacted by email using the membership mailing lists and asked to complete the survey and, if possible, spread the questionnaire to patients and family caregivers using posters and flyers. Healthcare professionals and volunteers were asked to explain the study and invite patients and family caregivers to participate during consultations or hospitalisations and make flyers available for use in waiting rooms and consulting rooms. No participant received any compensation for their participation.
Supplemental material
A multidisciplinary steering committee including board-certified PC physicians, methodologists, nurses, a sociologist, an anthropologist and an information specialist analysed all the questions collected in response to the questionnaire. They deleted duplicate questions, did a thematic and population classification and modified questions using the PICO (patient problem, intervention, comparison and outcome) format,33 34 performed a literature review for each question, searched for any existing systematic reviews or notified the highest level of evidence found in available articles (level of evidence graded according to the classification system of the French health authority (Haute Autorité de Santé)).35 For quantitative research questions, a question was considered as already answered if a systematic review exists and was published within the 3 years prior to this study. Unanswered questions were kept for the second stage of this study. Systematic reviews used PubMed (Medline), Web of Science and the Cochrane Library database. Other databases were used ad hoc for specific themes (social/psychology/legal). For qualitative research questions, systematic reviews are less frequent and therefore, articles were evaluated using the COnsolidated criteria for REporting Qualitative research (COREQ) guidelines.36 The COREQ is a 32-item list evaluating the quality of qualitative research studies. One point was given for each item present, to yield a score out of a maximum of 32 points. Studies with a score between 28 and 32 were classed as Grade A. All biases were noted. If an article was published within 10 years prior to this study and is classed Grade A according to the COREQ guidelines, then the qualitative research question was considered as already answered. Otherwise, the question was considered unanswered and kept for the second stage of this study.
All questions were reformulated and structured using the PICO framework to yield a coherent format for all the unanswered research questions retained.
The second stage of this study consisted of a first-round prioritisation using a fixed format questionnaire (online supplemental file 2) and lasted 6 months, from 15 January to 1 July 2024. The questionnaire was developed using the guidance from the James Lind Alliance and the UK public consultation ‘Palliative and End of Life Care Research Priorities Project’.21–23 25 29 The questionnaire was tested for readability and comprehension on patients and healthcare professionals, and 44 modifications were made. These modifications were deemed necessary due to misunderstandings about medical terms or specific concepts (art therapy/animal mediation). Patients were recruited in nine centres including seven teaching hospitals. Family caregivers and health professionals were invited to respond using the same methods as in the first step.
Supplemental material
The secondary objectives were to study differences in priorities between different subpopulations (eg, healthcare professionals, patients, family caregivers and volunteers) and to explore the transcultural nature of the questions according to geographical distribution.
The third stage of the study aims to reach a consensus on the top 10 unanswered questions in PC research, using the nominal group technique. The secondary objective is to study the reasons for that prioritisation. The method used is inspired by the James Lind Alliance and the UK public consultation ‘Palliative and End of Life Care Research Priorities Project’ and recommendations on how to conduct and analyse a nominal group.24 37 In order to enrol patients meeting the inclusion criteria, and in view of their physical condition, patients will be recruited from the Grenoble teaching hospital only, as will family caregivers. They will be contacted by email (for bereaved family caregivers) or during follow-up for current family caregivers. Concerning healthcare professionals, they will be invited to participate by email, distributed by the professional society for PC in France (ie, SFAP). During a 1-day meeting, patients, volunteers, caregivers and PC healthcare professionals will discuss, rank and reach a consensus on the propositions. To respect the inclusion criteria and patient frailty, the PC team from Grenoble will enrol all patients. During the 1-day meeting, 2 groups will be constituted, with equal representation from all participant groups. From the second stage of this study, the top 25 questions will be retained. The comprehensibility of the questions will be tested first. Clarifications will be made if needed. Each expert will evaluate the questions and rank them from 1 to 10, yielding a total score for each question in each group. Both groups will debate the reasons for their prioritisation. A first round of ranking will be performed. Then, groups will be changed, and another round of evaluating questions and debate will take place. A final prioritisation will be obtained, with a final ranking of the top 10 unanswered questions in PC research in France. Debates will be recorded and verbatim analysed in order to understand the reasons for the prioritisation. On the day of the nominal group gathering, discussions will be facilitated by two social psychologists trained in the nominal group technique.
Questionnaires
Both questionnaires for the first and second stages of this study will be made available online and created using Limesurvey. Answers will be anonymous. For the inclusion of patients, two strategies will be adopted: the first by dissemination of the questionnaire only, without any funding, and the second approach will enrol patients by funding inclusion centres to disseminate the questionnaire.
Posters and flyers with a QR code will be made available, and PC professionals will be asked to make them available to patients in their wards.
The first survey (online supplemental file 1) will ask respondents which themes they believe should be prioritised by PC researchers. Then, the second questionnaire will identify specific questions in each theme that need to be treated as a priority. To identify respondents, one question will be about their status: healthcare professional, social worker, patient, family caregiver, volunteer or ‘other’, with space for free text to specify their status under the heading ‘Other’. Respondents’ characteristics will be collected, including PC qualifications or years of PC experience for healthcare professionals and volunteers. Finally, respondents are asked to indicate how they heard about the survey, in order to garner information about the most effective method of dissemination.
The second survey (online supplemental file 2) will ask respondents to select questions of interest and then to rank the top 10, by attributing from 1 to 10 points, where 10 points are attributed to the most important question that should be prioritised first. Respondents’ characteristics will be collected. For patients, they are asked how they heard of the survey, and if they responded ‘inclusion centre’, they are asked to specify which centre.
Ethics
In accordance with current French legislation, Ethics Committee approval is not required for this type of study. This study is funded by Fondation de France, a private organisation funded by donations. No written consent will be required for the first and second stages of the study; participation is voluntary, there is no risk to respondents and the questionnaire responses are anonymous. Email addresses will be collected only if the respondent leaves them in order to be contacted after the first stage with a view to participate in the subsequent stages of the study. For the third stage of the study, discussions will be recorded and therefore, written informed consent will be requested from all participants. This study was registered with the French Health Data Hub under the number n°F20210719132105.
Statistics and data analysis
Questionnaires will be analysed using SPSS software (Chicago, Illinois, USA). Quantitative variables will be described using means±SD. Qualitative variables will be described as numbers and percentages. After transcription, all discussions will be recorded and analysed with the help of NVivo software, using thematic analysis, to understand the reasons underpinning the ranking of the most important questions. The aim of thematic analysis is to identify and categorise the different themes occurring in a cross-sectional manner across all interviews. Each theme is then considered as a meaningful and independent unit of the discourse. Major themes and secondary themes may be identified. Major themes are relevant points that are spontaneously well-developed by all participants. Minor themes are less well-developed by participants, seeming of lesser importance in their discourse and not necessarily mentioned by all participants. Data generated by the study will be stored on a secure server in the PC department of the University Hospital Grenoble-Alpes. Only authorised personnel involved in the study can access the data, which is password-protected. The data, and any copies thereof, will be deleted at the end of the study, in accordance with French legislation, that is, at a maximum of 2 years after the last publication of study results, or, in the absence of publication, 2 years after the validation of the final study report.
Discussion
Based on the results of the first step from patients and caregivers, two different enrolment strategies have been developed for the second step, namely: dissemination (identical to the first step) and inclusion by centres. Nine hospitals will be asked to include patients and family caregivers. Those hospitals (eight teaching hospitals and one general, non-academic hospital) were recruited based on their high volume of PC follow-up and availability of resources to participate in the present research project. To this end, we have obtained funding from Fondation de France and the Société Française d’Accompagnement et de Soins Palliatifs.
This study aims to produce a ranked list of the top 10 unanswered questions about PC in France. It will also evaluate a network collaboration. Indeed, both questionnaires will be disseminated using different strategies. We will be able to evaluate each network and describe how the information is disseminated, especially regarding the inclusion of patients and family caregivers. Indeed, the major risk of this study might be the low participation of patients and family caregivers. This is a current problem in PC research.38–40 That is why, in this study, two different strategies to enrol patients will be tested: first by questionnaire dissemination only, and second, by inclusion centres. It has emerged from the first step of the study, which is already completed, that trying to disseminate the questionnaires freely only via volunteers and word of mouth does not yield a large enough sample. Concerning healthcare professionals, questionnaires dissemination by different professional societies, the national PC research platform and PC teams should reach a majority of active PC healthcare professionals in the country.
As this study is inspired by the methodology of the public consultation performed in the UK, one would expect the number of participants to be similar, given that the population of France is about the same as that of the UK (approximately 67 million inhabitants). However, due to the reality observed in French research networks41 42 whereby participation in studies that provide no compensation is lower than in paid studies,43 half the number of participants is expected, namely around 1500 respondents for the first round and 800 for the second.
This study should provide new insights into how patients and caregivers prioritise themes such as euthanasia, assisted suicide and end-of-life laws. Indeed, in France, a bill is currently working its way through the legislature that proposes to legalise euthanasia and assisted suicide and will be voted on soon. Despite holding a Citizen’s Convention about the proposed legislation in 2023,44 no evaluation of the comprehension of the new law or the application of previous end-of-life laws45 was made.
Reaching a consensus about the top 10 unanswered questions will represent a major step forward in PC research for all stakeholders. Patients and family caregivers will have their opinions taken into account. Researchers will have to acknowledge patients’ priorities. Finally, a clear understanding of the reasons for the final prioritisation is essential. The last stage of this study will enable us to understand the reasons underpinning the ranking of each topic.
This study could also help to develop patient and family caregiver partnerships in future research. Despite the obvious benefits to researchers of involving patients in the development of studies, there are also several advantages for the patients themselves. Some of the benefits previously described include acquiring skills and gaining knowledge about research, gaining confidence in identifying themselves as experts and advocates, developing a social network of supportive peers and simply having a source of positivity.46 Establishing clear roles and working on power-sharing are fundamental to the coproduction of research work.47
Our study has some limitations, notably those inherent to the methods used. Indeed, this study is subject to selection bias due to the use of questionnaires and the enrolment methods deployed during the three steps. During the first step of our study, one major limitation was the low recruitment of patients and family caregivers, which led to disparities between groups and under-representation of patients and family caregivers. However, the second and third steps will use different recruitment methods, and it is therefore expected that a more representative number of patients and family caregivers will be enrolled.
Ethics statements
Patient consent for publication
Acknowledgments
First of all, to the patients. Thank you so much. To the SFAP: Société Française d’Accompagnement et de Soins Palliatifs. To the Plateforme Nationale de Recherche sur la fin de vie.
References
Footnotes
X @paquito130
Contributors CB: study concept and design/steering committee/statistical analysis/drafting of the manuscript/funding research/critical revision of the manuscript for important intellectual content. RH-D: study concept and design/steering committee/critical revision of the manuscript for important intellectual content. HR: study concept and design/steering committee. DH: study concept and design. RH: study concept and design. CT: study concept and design/acquisition of data/steering committee. MJ-C: acquisition of data/steering committee. VA-G: acquisition of data/steering committee. IL: acquisition of data, steering committee. JB: acquisition of data, steering committee. GE: critical revision of the manuscript for important intellectual content. FE: drafting of the manuscript/critical revision of the manuscript for important intellectual content. SS: study concept and design/statistical analysis, drafting of the manuscript/critical revision of the manuscript for important intellectual content. CB is the guarantor.
Funding This study is funded by Fondation de France, a private organisation funded by donations. The amount is €26 000. This study is funded by the Société Française d’accompagnement et de soins palliatifs. The amount is €5000. Those funders were not involved in the study design or having any implication in the study whatsoever.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design or conduct or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.