Article Text
Abstract
Introduction Malaria is still a major health problem in sub-Saharan Africa, where 98% of global malaria mortality occurs. In addition, the spread of Plasmodium falciparum with partial artemisinin resistance in East Africa and beyond is a great concern. The establishment of more effective vector control, in addition to the current long-lasting insecticide-treated net distribution programme, is an urgent task in these areas. One novel vector control candidate is the pyrethroid-PBO ceiling nets (OlysetPlus ceiling nets) which can overcome the problems of variations in net use behaviours and metabolic resistance to insecticide in vectors. Our preliminary study suggests the protective efficacy and high acceptability of this tool. With this proposed second trial, we aim to evaluate the impact of this tool in a different eco-epidemiological setting in the lake endemic region of Kenya.
Methods A cluster-randomised controlled trial is designed to evaluate the impact of pyrethroid-PBO ceiling nets in Ndhiwa Sub-County, Homa Bay County, Kenya. A total of 44 clusters will be randomly assigned in a 1:1 ratio to the intervention group (pyrethroid-PBO ceiling nets) and the control group. The assignment will be accomplished through covariate-constrained randomisation of clusters. For the primary outcome of clinical malaria incidence, 38 children from each cluster will be enrolled in a cohort and followed for 18 months. We will also evaluate the effects of the intervention on entomological indicators as well as its acceptance by communities and cost-effectiveness.
Ethics and dissemination Ethics approvals were provided by the Mount Kenya University Institutional Scientific Ethics Review Committee and the Ethics Committee Osaka Metropolitan University. Study results will be shared with study participants and communities, the Homa Bay County government and the Kenya National Malaria Control Programme. Results will also be disseminated through publications, conferences and workshops to help the development of novel malaria control strategies in other malaria-endemic countries.
Trial registration number UMIN000053873.
- Malaria
- Mosquito Vectors
- Infection control
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is a second cluster-randomised controlled trial of a novel vector control tool, pyrethroid-PBO ceiling net, to evaluate its efficacy in reducing malaria incidence among children.
The implementation of monthly active screening within the prospective cohort population established in each cluster facilitates the assessment of infection incidence.
The incorporation of multidisciplinary outcomes, encompassing social aspects and cost-effectiveness analyses, provides valuable insights for the potential future deployment of this intervention within integrated malaria control strategies.
One of the anticipated limitations is the possible contamination between intervention and control clusters because we will not set a buffer zone due to the geographical proximity among clusters.
Introduction
Background and rationale
Malaria is still a major health problem, particularly in sub-Saharan Africa, where 98% of global malaria mortality occurs.1 Although the morbidity and mortality of malaria declined from the 2000s to 2015 owing to many investments and interventions, such as long-lasting insecticide-treated nets (LLINs), malaria rapid diagnostic tests (RDTs), and artemisinin-based combination therapies (ACTs), progress has stalled since 2015. Moreover, the spread of Plasmodium falciparum partially resistant to ACT in Africa is an enormous concern. Currently five African countries—Rwanda,2 Uganda,3 Eritrea,4 Ethiopia5 and the United Republic of Tanzania6—have reported delayed clearance of P. falciparum after treatment with ACTs. Kenya’s proximity to these countries highlights the urgent need to establish effective vector control, in addition to maintaining antimalarial drug efficacy and strengthening resistance surveillance.
Among several vector control measures, LLIN is the most widely adopted tool to prevent mosquito bites and interrupt malaria transmission. However, suboptimal uses of LLIN are one of the key factors in reducing the impact of LLIN on the malaria burden, together with insufficient provision in the mass net distribution programme or shortening durability of nets.7 In the Lake Victoria basin, alternative uses of LLIN for fishing and protecting crops and chicks are well-known local behaviours8 9 as reported in other endemic areas.10 In fact, in many areas including our study sites in Homa Bay County, Kenya, malaria prevalence remains high despite widespread distribution of LLINs and their periodic replacements for more than a decade. This suggests that LLIN alone is insufficient to interrupt malaria transmission in this region.
Recently, we have proposed a novel vector control tool that covers the ceiling and the gap between the ceiling and the walls of residential structures with co-formulated pyrethroid and piperonyl butoxide (PBO) bed net material, called the OlysetPlus (Sumitomo Chemical) ceiling net. The benefit of installing the pyrethroid-PBO ceiling net in addition to conventional LLINs is detailed elsewhere.11 Briefly, the pyrethroid-PBO ceiling net provides a combination of physical and chemical protection against mosquitoes which seek human bloodmeal in the house. Recent reports have demonstrated that a substantial proportion of residual biting exposure occurs between the hours of entering indoor spaces and retiring to bed,12 pyrethroid-PBO ceiling nets may have a significant impact. Furthermore, the ceiling net is semipermanently installed and requires no further action from end users, thus its protective efficacy is consistently extended to all who stay in the house and less affected by factors such as discomfort or sleeping arrangement that contribute to variations in conventional LLIN use.13 The concept of ceiling net was previously investigated,14 and in this study, by integrating it with pyrethroid-PBO bed net material, this tool is anticipated to be effective even against pyrethroid-resistant mosquitoes, which are widely reported across Africa.
The aim of this study is to evaluate the efficacy, acceptability and cost-effectiveness of pyrethroid-PBO ceiling nets on malaria morbidity and transmission in the Lake Victoria basin of Kenya. Preliminary data from our previous study on Mfangano Island in Lake Victoria11 suggest a substantial reduction in malaria prevalence among school children and high community acceptance of this tool (unpublished data). With this proposed second trial, we aim to evaluate the impact of this tool in a different eco-epidemiological setting with relatively higher malaria transmission, more frequent human and vector movement, and synergistic impact from other interventions such as indoor residual spraying (IRS) and the RTS,S malaria vaccine. Since effective malaria controls need to be tailored to the local context, evidence of the effectiveness of pyrethroid-PBO ceiling nets from various transmission settings will increase the appeal of this intervention. Furthermore, considering the recent increase in choices of malaria control tools and the necessity of combining various tools to maximise the impact of the malaria control programme, it is important to understand the acceptability and cost-effectiveness of each intervention to guide its future deployment.
To achieve these objectives, our collaboration with local institutions, including the Kenya National Bureau of Statistics, the National Malaria Control Programme (NMCP), the Kenya Medical Research Institute and Homa Bay County, started from the research planning stage. This collaboration is crucial to the seamless transition from field trial to expanded implementation and policy development.
Objectives
The study has four research domains: epidemiology, entomology, social aspects and cost-effectiveness.
For the epidemiology domain, the primary objective is to determine the protective efficacy of pyrethroid-PBO ceiling net in reducing malaria clinical incidence in children 6 months to 14 years old over 18 months post intervention. This age range was selected to include both children under 5 years who are at high risk of malaria-related morbidity and mortality, and school-age children who have the highest prevalence of Plasmodium infections.15 The secondary objectives are to (1) determine the protective efficacy of pyrethroid-PBO ceiling nets in reducing Plasmodium infection prevalence by PCR in all age groups at 6, 12 and 18 months post intervention; (2) determine the spillover effects of pyrethroid-PBO ceiling nets in reducing Plasmodium infection prevalence in all age groups at 6, 12 and 18 months post intervention; and (3) determine the protective efficacy of pyrethroid-PBO ceiling net in reducing Plasmodium infection incidence in children 6 months to 14 years old over 18 months post intervention.
For the entomology domain, the primary objective is to evaluate the impact of pyrethroid-PBO ceiling nets on the indoor mosquito density of the primary malaria vector species captured by Centers for Disease Control and Prevention miniature light traps (CDC light traps). The secondary objectives are to (1) determine the impact of pyrethroid-PBO ceiling nets on the entomological inoculation rate (EIR) and (2) determine the prevalence of voltage-gated sodium channel (VGSC) mutations in vectors.
For the social aspects domain, the primary objective is to assess the determinants of social acceptability of the pyrethroid-PBO ceiling net in both the intervention and control arms. The secondary objectives are to (1) determine the feasibility of installing the pyrethroid-PBO ceiling nets and (2) measure attitudes, emotions, knowledge and beliefs relating to the ceiling net in Ndhiwa Sub-County.
For the cost-effectiveness domain, the primary objective is to determine the incremental cost-effectiveness ratios of adding the pyrethroid-PBO ceiling net to existing malaria control interventions under field trial conditions. The secondary objectives are to (1) establish the relative contribution to costs of the distinct programmatic elements and identify the inputs that contribute the most to overall costs, and (2) estimate the potential cost of providing pyrethroid-PBO ceiling net at a larger scale over 3 and 5 years under operational scenarios.
Trial design
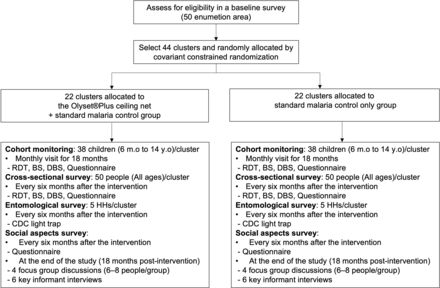
The study is an open-label, cluster-randomised controlled trial (CRCT) with 44 clusters evenly divided between the intervention and control arms. Each cluster will include one or two villages and consist of at least 50 households. A baseline survey will be conducted to determine the preintervention Plasmodium prevalence and Anopheles density, and collect demographic and socio-economic data for covariate-constrained randomisation of clusters. The baseline survey will be conducted 1 month before cluster randomisation. The postintervention follow-up period will be 18 months. For the evaluation of the primary objective, 38 children aged 6 months to 14 years from each cluster will be recruited and followed for 18 months as a cohort. Cross-sectional surveys will be conducted after 6, 12 and 18 months of the intervention targeting 50 individuals of all ages from each cluster to estimate the overall Plasmodium prevalence.
Methods: participants, interventions and outcomes
Study setting
Location and administrative structure
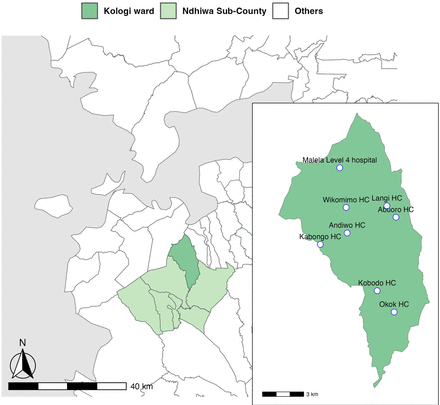
Ndhiwa (713.5 km²) is one of nine subcounties in Homa Bay County in Kenya. The subcounty has seven administrative wards: Kanyamwa Kologi, Kanyamwa Kosewa, Kabuoch North, Kwabwai, Kanyadoto, Kanikela and Kabuoch South/Pala. Based on the number of malaria cases reported in the Kenya Health Information System (KHIS), the accessibility of the site and population size, we selected Kanyamwa Kologi Ward as the target area (figure 1). Agriculture is the primary economic activity, with sugarcane as a main commercial crop. County residents also keep animals such as dairy cattle, beef cattle, sheep, goats and poultry.16 The ward experiences a long rainy season from March to June and a short rainy season from October to December. As of 2019, the average monthly precipitation was 228.64 mm and the mean annual temperature was 26.7℃. The relative humidity remains elevated year-round, fluctuating between 75% and 85%.17
Map of the study area. Inset shows the locations of the level 4 hospital and seven health centres) in Kanyamwa Kologi Ward.
Demographics
The population of Kanyamwa Kologi Ward is approximately 33 000 according to the 2019 national census.18 The dominant ethnic group in the region is Luo, and the primary languages are DhoLuo, Kiswahili and English. There are 172 primary and 36 secondary schools in Ndhiwa Sub-County.19 Within Kanyamwa Kologi Ward, there are 28 primary and 7 secondary schools.
Malaria epidemiology and control measures
Based on the KHIS, there were 429.1 and 457.2 confirmed malaria cases per 1000 population in Ndhiwa Sub-County and Kanyamwa Kologi Ward, respectively, in 2023. The primary malaria vector in the subcounty is Anopheles funestus, which prefers feeding on humans. Although Anopheles arabiensis exhibits predominantly zoophilic behaviour, it is also a significant malaria vector in our study area.20 In Homa Bay County, LLINs have been distributed every 3 years since the early 2000s, and IRS and the RTS,S malaria vaccine have been piloted in several areas since 2018 and 2019, respectively.21 Notably PBO-incorporated LLINs (VeeralinLN, manufactured by VKA Polymers, Tamil Nadu, India) were distributed in late 2023. In Kanyamwa Kologi Ward, there is one level 4 hospital and seven health centres.
Eligibility criteria
As the ceiling nets are installed per structure, we set the inclusion criteria on a structural basis. The inclusion criteria for the installation of the ceiling nets are (1) residential structures with at least one permanent resident aged 18 years or older in the household, (2) informed consent provided by a resident in the household and (3) house structure amenable to ceiling net installation in terms of size of the structure, presence of eave, ceiling board and vertical beams, and material of the top part of the wall. The applicability of the ceiling net installation will be assessed by experienced field staff. The exclusion criteria are (1) vacant structure, confirmed by at least two visits by community health promoters (CHPs), (2) dwelling structure to be vacated or destroyed within the study period, (3) non-eligible house structure for the ceiling net installation and (4) non-residential structures (school, shop, kitchen, storage and toilet). The inclusion criteria for the prospective cohort are (1) children aged 6 months to 12 years old at the time of enrolment, (2) living in the study area at the time of pyrethroid-PBO ceiling net installation, (3) having no plan to leave or stay outside the study area for an extended period (longer than 1 month) over the 18-month follow-up period and (4) informed consent provided by the participants or the parent or legal guardian. The exclusion criterion is having severe chronic illnesses. The inclusion criteria for cross-sectional malaria surveys for all age groups are (1) living in the study area during the study period and (2) informed consent being provided by the participants or the parent or legal guardian before each survey. The exclusion criteria are (1) having severe chronic illnesses and (2) pregnancy known at the time of the surveys.
Who will take informed consent?
Written informed consent will be obtained by the study team members who fully understand the study protocol. After eligibility is confirmed, the study team members will present to the potential participant a document containing all relevant information about the study in Luo and English. If the participant cannot read, study information will be conveyed verbally in Luo, Kiswahili and/or English by the study team members. The potential participant will have opportunities to ask any questions. Agreement to participate will be sought only after the participant indicates complete understanding of the study.
Additional consent provisions for collection and use of participant data and biological specimens
The study information document for ceiling net installation contains the study overview. In addition, the documents for cross-sectional and cohort surveys contain details on collecting, storing and using personal data and biological specimens during the study.
Interventions
Explanation for the choice of comparators
In Kenya, LLIN is the most widely used malaria preventive measure. The Division of National Malaria Programme coordinates free LLIN distribution, and the county governments deliver LLINs to residents in all endemic counties every 3 years. In Homa Bay County, the RTS,S malaria vaccine has been implemented since 2019. The primary purpose of this trial is to demonstrate the superiority in malaria prevention of adding pyrethroid-PBO ceiling nets to the standard malaria control programme. Thus, in the control arm, no pyrethroid-PBO ceiling nets will be installed, but LLIN use and RTS,S immunisation will be allowed in the control and intervention arms as the current best practice. There is no plan for new LLIN distribution during the study period.
Intervention description
In the intervention arm, pyrethroid-PBO ceiling nets will be installed in all dwelling units where residents sleep, free of charge to the households. All participants will be encouraged to continue to use LLINs, distributed by the Homa Bay County government. In each intervention cluster, one CHP and two community volunteers will be recruited from the intervention cluster and another CHP from the control cluster will join the team to enable future knowledge dissemination. The net installation team will be trained to instal ceiling nets by skilled local research assistants who participated in previous trials. The head (or another adult) of the household eligible to a ceiling net will be notified at least 24 hours before the scheduled installation time. The cost of the ceiling nets and their installation will be covered by the research team. Details of the installation procedure are described in a previous study protocol.11 Briefly, the ceiling net is a rectangular sheet of pyrethroid-PBO net with loops sewn along the diagonal seams. The loops are roped to the support beams under the roof and the edges of the net are stapled to the wall.
Criteria for discontinuing or modifying allocated interventions
As the ceiling net is semipermanently installed, the intervention will only be discontinued if the participant specifically requests the removal of the ceiling net by the study team. There will be no crossover from the control arm to the intervention arm during the follow-up period. Those who migrate between the arms or emigrate from the study areas will be dropped from the study follow-up.
Strategies to improve adherence to interventions
Adherence to the intervention cohort in this study is defined as sleeping in houses with pyrethroid-PBO ceiling nets. Adherence is monitored indirectly by assessing the number of nights each participant spends outside their house during the monthly interview. During each house visit, CHPs will visually inspect the condition of the ceiling nets. Any visible tear and damage to the ceiling net will be reported to the research team, who will assess the size and location of the damage and perform repair or replace the ceiling net if necessary.
Relevant concomitant care permitted or prohibited during the trial
There is no specific concomitant care prohibited during the trial. All participants in both arms will continue to receive and use free LLIN and have access to standard medical care, including malaria testing by RDT, treatment with ACT and RTS,S malaria vaccination.
Provisions for post-trial care
All participants will be under the normal healthcare system in the study setting. No perceived health risks for the intended population are expected with the intervention. To monitor the long-term impact of the intervention, the research team may conduct additional cross-sectional surveys 2 and 3 years post intervention to monitor further parasite transmission in the population.
Outcomes
Epidemiological domain
The primary outcome will be symptomatic malaria case incidence, defined as axillary temperature of ≥37·5°C or a history of fever in the preceding 48 hours, and positive RDT, in children aged 6 months to 14 years enrolled in the cohort, monitored with a monthly visit and passive case detection in the health facilities during an 18-month follow-up. The secondary outcomes will be (1) the prevalence of Plasmodium infections by PCR in all age groups at 6, 12 and 18 months post intervention and (2) infection incidence by PCR in the prospective cohort of children aged 6 months to 14 years over 18 months.
Entomological domain
The primary outcome will be the density of the primary malaria vectors, species composition and sporozoite infection rates. Indoor malaria vector density will be determined using CDC light trap, and species composition and sporozoite infection rates will be determined by microscopy and PCR. The secondary outcomes of the entomology domains will be (1) changes in EIR as a measure of malaria transmission and (2) prevalence of VGSC mutations associated with insecticide resistance in Anopheles mosquitoes captured by light trap.
Social aspect domain
The primary outcome will be the percentage of households consenting to pyrethroid-PBO ceiling net installation when offered. In addition, we will include observations and discussions about individual attitudes towards the ceiling net. The secondary outcomes of the social aspect domain will be the percentage of the intact ceiling net, description of damaged net and the impact on the living environment such as perceived temperature in the house, dirt/debris trapped by the ceiling net, loss of storage space at the top of the wall and rewiring of power lines. They will be evaluated by questionnaires at 6, 12 and 18 months post intervention.
Cost-effectiveness domain
The primary outcome will be the incremental cost-effectiveness of adding pyrethroid-PBO ceiling net to existing malaria control interventions under field trial conditions from the societal and provider perspectives. The secondary outcomes of the cost-effectiveness domains will be (1) the costs of the distinct programmatic elements and the inputs that contribute the most to overall costs, and (2) the cost of providing pyrethroid-PBO ceiling net at a larger scale over 3 and 5 years under operational scenarios.
Participant timeline
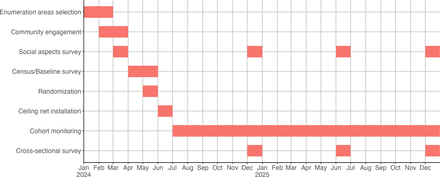
The schedule of trial activities is presented in figure 2. The detail of each survey is described in figure 3.
The schedule of trial activities.
Consolidated Standards of Reporting Trials flow diagram and the detail of each survey. RDT, rapid diagnostic test.
Sample size
The sample size was calculated using Hayes and Moulton’s method.22 All sample sizes will be recalculated based on the baseline data, which will be collected about 1 month before the ceiling net installation.
Epidemiological survey
The following calculations were based on the historical data collected from the Lake Victoria region in Kenya with a clinical malaria incidence rate of 0.5 per person-year in children under 14 years old by RDT (unpublished data on Mfangano island), 40% parasite prevalence for all age groups by PCR and a between-cluster coefficient of variation (CV) in incidence rate of 0.24 in both groups. In the study site, RTS,S malaria vaccination began in 2019, with a mass distribution of PBO-incorporated LLINs at the end of 2023. Therefore, the intervention effect is expected to be smaller than those in previous studies and is conservatively assumed to be 25%. Assuming 38 individuals per cluster to be followed for up to 18 months with 20% loss-to-follow up rate, we require 22 clusters per arm, a total of 1672 children, to achieve 80% power to detect a significant incidence rate ratio of 0.75 (25% protective efficacy) at a two-sided type 1 error of 5%. With 50 individuals per cluster (2200 total individuals) for the secondary outcome of Plasmodium prevalence by PCR in all age groups, we would achieve 5% type 1 error and 80% power to detect 23.5% relative reduction. We do not specify the sample size for identifying spillover effects because spillovers tend to have smaller effect sizes relative to total or overall effects, so typically larger sample sizes are required to detect them. Although our study may be underpowered to detect spillovers, we will report the results as an exploratory analysis.
Entomological survey
Based on the previous entomological study conducted in Homa Bay County, we assume a mean vector density (number of mosquitoes per CDC light traps) of 3.3, an SD of 0.634 and CV of 0.192.14 For 80% power to detect a 50% decrease in mean mosquito densities at 5% type 1 error level, we need to capture mosquitoes from five houses in each of the 44 clusters.
Recruitment
38 eligible children in each cluster will be randomly recruited into our cohort. Recruitment will be limited to children aged 12 or younger to avoid children ageing out during the 18-month monitoring period. Study team staff will obtain informed consent from the parents or caregivers of the children before enrolling the children in the cohort. For the cross-sectional survey at each time point, we will randomly select from each cluster 50 individuals of all age groups. To guarantee the representativeness for all age groups, the selection will be done with the following age category stratifications: 0–4, 5–9, 10–14, 15–19 and 20 and above.
Assignment of interventions: allocation
Sequence generation
Random numbers will be generated using the sample function in R software.
Concealment mechanism
The individual, household and villages (clusters) are all given unique IDs at the beginning of the baseline. Any following steps handle only these anonymised IDs.
Implementation
After the baseline survey, covariate-constrained randomisation will be used to allocate the 44 clusters across the two study arms. The following factors will be constrained: baseline malaria infection prevalence by RDT in children aged 0.5–14 years, LLIN usage, malaria vaccine coverage, socio-economic status (SES), population size, the proportion of eligible houses for the ceiling net installation and vector densities. An independent statistician will perform the randomisation. Local study assistants will perform participant enrollment.
Assignment of interventions: blinding
Who will be blinded
Due to the visibility of the pyrethroid-PBO ceiling net, neither the trial participants nor the members of the study team who take part in field activities can be blinded. However, laboratory- and office-based personnel (eg, microscopists, laboratory technicians and data analysts) will be blinded to the identity and intervention status of the trial participants since all biological specimens will be identified by a unique numeric study identifier, and personal information will be removed before analyses.
Procedure for unblinding if needed
This is an open-label trial, and only the data measurers are blinded. Therefore, there is no circumstance that they need to be unblinded.
Data collection and management
Questionnaires for the baseline survey, cohort surveys and postintervention cross-sectional surveys are provided in the online supplemental files 1–4.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Plans for assessment and collection of outcomes
Census and baseline cross-sectional survey
In Kanyamwa Kologi Ward, 85 census enumeration areas (EAs) are defined by the 2019 Kenya Population and Housing Census. Births, deaths and migrations in each EA are regularly updated by CHPs using the integrated community health system maintained by the Ministry of Health. 50 EAs are randomly selected for our baseline survey, during which demographic information of all individuals is updated. To ensure balanced cluster allocation, the baseline survey includes a questionnaire for all households, RDT testing of all children aged 6 months to 14 years and an entomological survey of randomly sampled households. We modified the questionnaire used in the 2020 Kenya Malaria Indicator Survey mainly to quantify the SES of each household and bed net usage. In addition, we add questions to quantify the favourability of ceiling nets before the intervention.
Cohort monitoring
Incidence of clinical malaria in the prospective cohort will be estimated by both active and passive case detections. For active case detection, we will conduct home visits every 4 weeks. From all cohort participants, axillary temperature will be measured using a digital thermometer, and Plasmodium infection status will be determined by RDT and PCR. Participants with fever (>37.5°C) or other malaria-related symptoms listed in the Kenya National Malaria Treatment Guideline at the time of home visit or within the previous 48 hours will be tested for malaria by RDT. History of travel, confirmed malaria episode and visit to local health facilities since the previous visit are recorded. For passive case detection, we ask all cohort participants to visit designated health facilities in case they suspect malaria between home visits. The designated facilities are asked to record all malaria tests performed regardless of their results together with the cohort ID. The cost of RDT and antimalarial treatment will be covered by the research team to encourage cohort participants to use only designated facilities.
Cross-sectional malariometric surveys
Malaria prevalence in children and adults will be estimated using cross-sectional malariometric surveys in communities. These surveys will be conducted at 6, 12 and 18 months post installation. Community surveys will be conducted by house visits.
Plasmodium infection status will be determined using three methods: RDT, microscopy and PCR. A finger-prick blood sample will be collected for on-site diagnosis using the Bioline Malaria Ag P.f/Pan RDT (Abbott Diagnostics Korea Inc, Republic of Korea). Survey participants with positive test results will be provided with a treatment course of artemether-lumefantrine with dosing instructions in accordance with guidelines from the Ministry of Health in Kenya after checking their recent treatment history. Blood smears will be prepared on site and transported to the main laboratory in Homa Bay where thin smears are fixed with methanol and all smears are stained with 3% Giemsa solution for 30 min, then examined by experienced microscopists. Two blood samples (70 µL each) will be collected with a 75 mm heparinised micro-hematocrit capillary tube (Thermo Fisher Scientific, MA, USA) and spotted on Whatman ET31 Chr filter paper (Whatman International, Maidstone, UK). The blood samples will be allowed to dry at ambient temperature and stored in individual zipped plastic bags at −20℃. The dried blood spots will be used for the determination of malaria status by PCR.23
Entomological surveys
Indoor mosquitoes will be collected from five randomly selected houses within each cluster using the CDC light trap method. Samples will be preserved in 96% ethanol and placed in a cool box with ice. Specimens will be examined for sex determination by microscopy and species identification by microscopy and PCR. Indoor mosquitoes will be collected at baseline, 6, 12 and 18 months post-ceiling net installation.
Social aspects
We will conduct an exploratory sequential research design using integrated mixed methods (qualitative and quantitative). Qualitative assessment of community perceptions on the pyrethroid-PBO ceiling nets, community facilitators and concerns of pyrethroid-PBO ceiling net use will be implemented, followed by quantitative assessments every 6 months and routine monitoring to evaluate the durability of pyrethroid-PBO ceiling nets using observation checklists in randomly sampled houses. At the end of the study, other qualitative case studies, such as focus group discussions and key informant interviews, will be conducted to document any remarks related to the study and inform the sustainability and scalability of the intervention.
Cost-effectiveness analysis
Incremental financial and economic cost data of pyrethroid-PBO ceiling net will be collected alongside the intervention. In cases where resources, such as staff, are shared among multiple elements, the allocation of costs will be carried out using an appropriate proxy. Costs related to research activities will be excluded from this allocation. Financial costs will be derived from project expenditure records, while economic costs, which encompass financial expenditures and donated resources, will be identified through project records and social aspects activities. The value of donated resources will be credited based on prevailing market rates. Furthermore, capital costs will be annualised over their useful life for financial costing and annualised at a discount rate of 3% for economic costing.
Plans to promote participant retention and complete follow-up
All surveys planned for the epidemiological and entomological domains will be conducted by house visits. CHPs will make an appointment with eligible participants before each visit to confirm the participants’ available date and time. Small remunerations will be provided to survey participants to compensate for their time. CHPs will receive detailed instructions and participatory training for all field procedures and will be actively supervised by the research team throughout the duration of the study. Feedback will be regularly sought from CHPs regarding any issues raised by study participants, and discussions will be held to resolve issues from the field.
Data management
All data from the baseline, cohort and cross-sectional surveys will be captured using the Research Electronic Data Capture software on electronic tablets. Data will be uploaded daily to a highly secure server hosted by Mount Kenya University (MKU). All data from the quantitative surveys will also be stored securely and backed up regularly to prevent data loss. Data access and management of databases will be limited to authorised study investigators and collaborators. After validation of data uploaded to the MKU server, data stored locally on the tablet computers will be permanently deleted to minimise unauthorised access.
Confidentiality
To maintain confidentiality, each participant in cross-sectional surveys, the longitudinal cohort and the quantitative surveys is assigned a unique identifier. The data collected will be labelled using the unique identifier and stored separately from the key linking personal information (name, date of birth, GPS of each household and phone number). The data will be kept on a secure server that is only accessible to the research staff. Publications will contain only aggregated data, and no personal information will be included.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future use
Anonymised blood samples from study participants will be stored and analysed for Plasmodium infections by microscopy and PCR in our laboratory in Homa Bay. Microscopic examinations of adult mosquito specimens will be conducted in our field laboratory in Mbita, while PCR analyses will be conducted in our laboratories in Homa Bay and MKU. Laboratory data outputs will be entered in Microsoft Excel and imported into the database. No human genetic analysis is planned for this study. However, remaining biological materials will be stored indefinitely for future studies unless the participants opt out during the informed consent process. Participants are provided with contact information of the research team and can remove themselves from this study or any future studies at any time without penalty or prejudice.
Statistical methods
Statistical methods for primary and secondary outcomes
We will follow the Consolidated Standards of Reporting Trials guidelines extended for CRCT for statistical analysis and result reporting. The intention-to-treat analysis is the primary analysis approach for both the primary and secondary objectives for the epidemiological and entomological studies. The per-protocol analysis is included as a supplementary analysis for the primary and secondary objectives for the epidemiological and entomological studies. Detailed methodologies for the epidemiological part are described in the online supplemental file of statistical analysis plan.
Clinical malaria incidence
We will determine the protective efficacy of pyrethroid-PBO ceiling nets against malaria case incidence by comparing clinical malaria incidence rates between arms. We will use mixed-effects negative binomial regression accounting for within-cluster correlation of outcomes. Possible confounding factors such as age, sex, bed net usage, house structure, malaria vaccination history and SES will be adjusted as well as covariates used in the covariate-constrained randomisation. In addition, because we will not set a buffer zone, the distance to the nearest household in the other arm will be adjusted in the following analysis to reduce the contamination between the two arms. The variable was selected from the previous study.24
Prevalence of malaria infection
The secondary outcome, the prevalence of malaria infection by PCR and microscopy measured at 6, 12 and 18 months after the ceiling net installation will be analysed using mixed-effects logistic regression adjusting for the above-mentioned confounding factors.
Exploratory analysis for spillover effects
Evidence for positive spillover effects of the ceiling net on malaria infection prevalence in all age groups will be assessed by comparing individuals with no intervention conditioning (1) the distance to the nearest ceiling net installed household and (2) the coverage of surrounding households with ceiling net within 400 m. The distance of 400 m was chosen as the spillover effect appears to attenuate at this distance based on previous reports.25
Entomology
Differences in vector density and EIR between arms will be evaluated by random-effects negative binomial regression taking into account the intracluster correlation.
Social aspects
We will employ the Framework for Reporting Adaptations and Modifications to Evidence-based Implementation Strategies26 to document the implementation processes of the ceiling nets and the evidence integration triangle framework27 to align the evidence generated to policy and vector control strategies from the health systems aspect. The theoretical framework in qualitative research will be grounded theory.28 Data from ethnographic, focus group discussions and key informant interview will be summarised using content thematic analysis. Pre- and postintervention acceptability to instal pyrethroid-PBO ceiling net intervention will be compared with actual consent using logistic regressions.
Cost-effectiveness
The economic and financial costs associated with the pyrethroid-PBO ceiling net intervention will be presented in total and disaggregated forms, highlighting the relative contribution of each programme element to the overall programme costs. To facilitate comparisons with other malaria vector control interventions, the costs will be converted into cost per household and per person receiving the intervention annually. Various programme scenarios, such as different scales and durations, will be presented to estimate operational implementation costs. Compared with the control group, we will use the number of malaria cases averted in the pyrethroid-PBO ceiling net arm to calculate the Disability-adjusted life years (DALYs) averted using standard methods.
Interim analyses
No interim analysis is planned because neither the insecticide permethrin nor the synergist PBO as formulated in pyrethroid-PBO LLINs is known to pose significant health and safety risks.11 14
Methods for additional analyses (eg, subgroup analyses)
We will perform the same analysis for three age subgroups (≤59 months old; 5 years old to 14 years old; 15 years old or older) to examine if the effects of pyrethroid-PBO ceiling net differ by age groups. In addition, we plan to use other machine learning methods to estimate the conditional average treatment effect, such as causal forests—which extend random forest algorithms for causal inference29—and the super learner algorithm, an ensemble method that combines multiple predictive models to improve accuracy.30
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data
In the cohort, non-adherence to the intervention can be identified by monthly interviews. Participants who regularly sleep outside their homes will be removed from the analyses. The extent and patterns of missing data will be assessed once all data collection has been completed. If necessary, we will apply simple hot-deck imputation methods if the missing fraction for the covariate is <5% or appropriate multiple imputation approaches if the missing fraction for a covariable are ≥5%. If a non-ignorable portion of the subjects has missing values on a covariate (due to missing at random or missing completely at random), that covariate may be excluded in the model.
Plans to give access to the full protocol, participant-level data and statistical code
This article is the full protocol. The corresponding author will make the deidentified data sets or any future statistical code available on reasonable request.
Oversight and monitoring
Composition of the coordinating centre and trial steering committee
The sampling team, composed of CHPs and laboratory technicians, set up a day-to-day communication group and exchanged their experiences. A local management team of study investigators from Kenya and Japan also joined this, leading and advising the activities and monitoring the sample and data integrity. A monthly meeting will be held by the steering committee composed of all key researchers from Kenya and Japan, including the principal investigator (PI) and co-PI, to monitor the progress of the trial.
Composition of the data monitoring committee, its role and reporting structure
Our intervention is considered to be of a low-risk nature. The data safety monitoring committee will consist of two medical doctors who are independent of the project organisations and sponsor and have no competing interests. The primary responsibilities of this committee will be to periodically review self-reported adverse events derived from the monthly questionnaire in the cohort. All severe adverse events observed or reported during the study will be reported to the committee in a timely manner, and the committee will determine the relationship between the severe adverse events and the intervention. For additional credibility about study quality, the researchers will consult a third statistician, if necessary.
Adverse event reporting and harms
In addition to the safety monitoring committee, researchers will compare self-reported non-serious adverse events such as coughs, rashes and itches between the intervention and the control arms in the cohort and cross-sectional surveys. In the event of a study-related serious adverse event, the study team will convene a meeting immediately with the MOH and Homa Bay County Teaching and Referral Hospital representatives to review the case and take necessary action.
Frequency and plans for auditing trial conduct
A monthly meeting will be held during the follow-up period to ensure that all surveys and investigations are conducted according to the study protocol. The study is required to submit annual reports and renewal to ethical review boards of Osaka Metropolitan University, Japan, and MKU, Kenya.
Plans for communicating important protocol amendments to relevant parties (eg, trial participants, ethical committees)
Decisions on important trial amendments must be made through a formal procedure and will be approved by institutional review boards at MKU and Osaka Metropolitan University. The protocol in the clinical trials registry will also be updated accordingly.
Dissemination plans
Study results will be shared with the study participants and communities, the Homa Bay County government and the Kenya NMCP. Results will also be disseminated through publications, conferences and workshops to help the development of novel malaria control strategies in other malaria-endemic countries. Suggestions from the participants will also help shape the future improvement of the intervention.
Ethics approvals
Ethics approvals were received from the Mount Kenya University Institutional Scientific Ethics Review Committee (MKU-ISERC) (approval number: 2565) and the Ethics Committee at Osaka Metropolitan University (approval number: 2024-068). Written informed consents will be sought from study participants before the baseline survey, installation of ceiling nets, each cross-sectional survey and the start of prospective cohort surveys. In cross-sectional and cohort surveys, informed assent will be obtained from children under the age of 15 who can understand the study at a level appropriate for their development, in addition to the consent of a parent or legal guardian. Participants have the right to withdraw from the study at any time and the option to withhold previously collected samples from any future analyses and studies. The samples collected in this study may potentially be used for other research purposes. This is clearly stated in the informed consent form. In such cases, we will obtain the necessary ethical approval and provide participants with the chance to opt-out from this. All experiments will be carried out in adherence to WHO requirements and the Declaration of Helsinki.
Discussion
Global malaria progress has flatlined in recent years: targets of reductions in malaria morbidity and mortality and required funding by 2030 are all off track as of 2023.6 In addition, P. falciparum with partial artemisinin resistance, which has been a problem in the Great Mekong Subregion for more than a decade, is emerging independently in sub-Saharan Africa.2–6 31 Novel interventions that are cost-effective and widely accepted by local communities are urgently needed to contain the spread of artemisinin-resistant P. falciparum in sub-Saharan Africa.
Early results from our CRCT of pyrethroid-PBO ceiling nets on Mfangano Island in Lake Victoria, Kenya, suggest that ceiling nets can reduce Plasmodium prevalence and are positively received by the local communities. Nevertheless, there are regional differences in housing design, vector abundance and composition, and availability of malaria control interventions. As such, the feasibility and acceptability of the ceiling net intervention are likely to depend on local eco-epidemiological context.32 Furthermore, one of the secondary objectives in this study is to measure the spillover effects, that is, how much a household that does not have a ceiling net benefits from living near a house with a ceiling net. This enables a broader understanding of the impact of the ceiling nets at the community level.
This trial has several limitations. First, although the study is designed as a CRCT, contamination between intervention and control clusters cannot be excluded as buffer zones between intervention and control clusters cannot be created due to geographical proximity of houses and villages in the ward. A recent study, however, has shown that the spillover effect of interventions on malaria can extend to 3 km,33 so buffer zones of a few hundred metres, as set out in many studies, may not be sufficient. We will try to eliminate such contamination effects by integrating spatial data into our statistical model. Second, because of the visible nature of the ceiling net, we cannot exclude open-label and observer biases. It is conceivable that participants receiving ceiling nets may reduce their usage of conventional LLIN as both interventions are made of the same materials and may be perceived to protect against malaria in the same manner. We aim to reduce such bias as much as possible through repeated reminders by CHPs that ceiling nets serve as an addition to and not a replacement of conventional LLINs. We will conduct surveys and in-depth interviews to elicit participants’ perceptions of the ceiling net, which can guide future messaging and implementation. To reduce observer bias, laboratory investigators and data analysts will be blinded. Third, in the study area, pyrethroid+PBO-incorporated LLINs were distributed in 2023. It may reduce the effect of our ceiling net intervention because pyrethroid+PBO-incorporated LLINs are more effective than non-PBO-incorporated LLINs by targeting both Anopheles vectors with and without metabolic resistance to pyrethroids. Pyrethroid+PBO-incorporated LLINs received a conditional endorsement from the WHO in 2017, and approximately half of the LLINs distributed in sub-Saharan Africa in 2022 were of this type.6 Given the abundance of PBO-incorporated LLINs in the region, it is important to assess the effectiveness of the pyrethroid-PBO ceiling net as an addition to these LLINs to inform policy recommendations. Recently LLINs combining two different classes of insecticides have been shown to be superior to pyrethroid-based LLINs.34 When these new LLINs become widely available, the effectiveness of pyrethroid-PBO ceiling nets needs to be reinvestigated.
Trial status
The baseline survey was started on 8 April 2024. The recruitment of the intervention participants and the ceiling net installation were conducted in June 2024. The current protocol is version 6.0 as of 11 November 2024.
Supplemental material
Ethics statements
Patient consent for publication
Acknowledgments
We would like to express our sincere gratitude to this study’s participants, field and laboratory staff. In addition, we acknowledge the collaboration and support of health offices in Homa Bay County, Kenya.
References
Footnotes
JG and AK are joint senior authors.
X @YuraKKo3
Contributors AK and JG are co-principal investigators. AK is the guarantor. YKK, WK and AK developed the original concept. All authors discussed and contributed to the study protocol. YKK, WK, PO, KBM, JK, VO, JO, SM, KNBS, KK, ES, GOO and AK contributed to the preparation of the baseline survey. YKK, WK, PO, KBM, TO, CWC, JK, SM and MK drafted the manuscript. YKK, WK, CWC, MK, GOO and JG contributed to the revisions of the draft of the manuscript. DY participated as a senior statistician. YKK and MK drafted the statistical analysis plan (SAP) and WK, CWC and DY revised it. The authors read and approved the final manuscript and SAP.
Funding AK and JG received support from JICA/AMED joint research project (SATREPS) (Grant no. 20JM0110020H0002), Hitachi Fund Support for Research Related to Infectious Diseases, and Sumitomo Chemical Corporation. The funding bodies play no role in the study design, data collection, analysis, interpretation, and publication.
Map disclaimer The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests AK and JG were partially supported by a research grant from Sumitomo Chemical Corporation. Other authors had no competing interests.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.