Article Text
Abstract
Introduction Despite its therapeutic advantages, postmastectomy radiotherapy (PMRT) increases the risk of complications and often leads to poor cosmesis in women undergoing breast reconstruction. Preoperative radiotherapy followed by skin-sparing mastectomy and deep inferior epigastric perforator (DIEP) flap reconstruction is technically feasible, with low rates of surgical complications and good short-term oncological outcomes. Further evaluation in a randomised trial comparing preoperative radiotherapy versus conventional PMRT in breast reconstruction is required to assess both oncological and patient-reported outcomes (PROs).
Methods and analysis The CAPPELLA trial is a prospective, multicentre, open-label, randomised controlled trial across nine centres comparing PROs and safety outcomes between preoperative and postoperative radiotherapy in patients with locally advanced breast cancer requiring immediate DIEP flap reconstruction. Female patients aged >18 years with breast cancer who are treated with neoadjuvant systemic treatment, require both mastectomy and radiotherapy and are suitable for DIEP flap reconstruction will be included. Patients will be randomly assigned (1:1) to a preoperative radiotherapy group or a postoperative radiotherapy group. Stratification will be performed by cancer centre at initial diagnosis. The radiation volumes will include the ipsilateral breast/chest wall, supraclavicular lymph nodes, undissected axilla and internal mammary nodes. The dose regimen will be 42.56 Gy in 16 fractions. The primary endpoint will be satisfaction with the breast domain of the BREAST-Q at 2 years postoperatively. The secondary endpoints will include PROs at 3, 12 and 24 months postoperatively in both groups, aesthetic assessment, complication rates, rates of total pathological complete response (tpCR) and tumour safety. All patients will be followed up for 36 months postoperatively. The app software will be used to collect all data prospectively. Data will be analysed using SPSS and Stata software. The target sample size will be 80 participants.
Ethics and dissemination This study will be performed according to the Helsinki Declaration. All patients will be asked to provide informed consent before enrolment. Approval for this study was provided by the independent ethics committee and institutional review board of Fudan University Shanghai Cancer Centre. We will present the study results at national and international meetings and publish them in a scientific peer-reviewed journal.
Trial registration number NCT05512286.
- Breast tumours
- Radiation oncology
- Patient Reported Outcome Measures
- Patient Satisfaction
- Breast surgery
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The use of patient-reported outcomes (PROs) in monitoring patient satisfaction and complications could increase patient engagement in cancer treatment, which is in line with the objectives emphasised in certain countries.
This study will also establish standards for the indicators, risk and prognostic value of immediate breast reconstruction and the timing of radiotherapeutic interventions and will provide suggestions for future clinical practice and research.
Heterogeneity in radiotherapy between centres may affect cosmetic outcomes.
The follow-up period of this study will be 36 months, which is sufficient for capturing PROs in patients undergoing breast reconstruction but relatively short for assessing prognosis.
Introduction
With increasing evidence supporting the oncological safety of immediate reconstruction and advancements in reconstructive techniques, an increasing number of patients have opted for breast reconstruction in recent years.1 In the USA, the reconstruction rate has increased from 14.8% in 2000 to 31.9% in 2011,2 and the application of immediate breast reconstruction (IBR) has nearly doubled over the last two decades.3 Moreover, patients who undergo IBR are more satisfied with their breasts and report several benefits, including in terms of psychosocial and superior aesthetics.4
In women with locally advanced breast cancer (LABC), breast reconstruction and postmastectomy radiotherapy (PMRT) decrease local-regional recurrence and improve survival in patients with node-positive disease.5–7 However, PMRT is associated with a high rate of surgical complications and reconstruction failure among patients who undergo IBR.8 9 Capsular contracture is the most significant long-term risk of implant irradiation. It can result in poor cosmesis, pain and discomfort for the patient. Similarly, complications of autologous reconstruction with PMRT may include poor wound healing, fibrosis, fat necrosis and flap shrinkage.10 Regardless of the reconstructive technique, there is a concern that IBR could affect the technical delivery of radiation therapy, with the largest compromises observed in patients with left-sided cancers.11 The optimal integration of breast reconstruction and PMRT has long been a challenge in oncoplastic breast surgery.
Although not entirely resolved, in a 2017 study based on the National Cancer Database, the number of patients who underwent IBR significantly increased from 13% in 2004 to 33% in 2013 in the setting of PMRT.12 Patients who received PMRT had fewer complications (OR=0.47, 95% CI=0.27 to 0.82, p=0.007) with autologous reconstruction and higher BREAST-Q breast satisfaction scores (63.5 vs 47.7; p=0.002) compared with implant-based Breast Reconstruction (BR).13 Many surgeons in the 2023 Oncoplastic Breast Consortium (OPBC) consensus conference preferred autologous reconstruction over all implant-based reconstruction options in the setting of PMRT.14 To minimise the risk of complications, plastic surgeons typically suggest postponing reconstruction in cases where autologous reconstruction is desired and when PMRT may be necessary. However, delayed reconstruction is associated with a lower health-related quality of life (QoL) compared with IBR.4
To reduce the negative effects of radiation on autologous breast reconstruction, there has been a growing interest in utilising neoadjuvant radiation therapy (NART). Recent studies have reported that NART is a proven strategy for improving cosmetic outcomes and simplifying the reconstructive process.15–17 Singh et al conducted a systematic review comprising 10 retrospective and eight prospective studies analysing the effects of NART on IBR. The reviews revealed overall complication rates ranging from 3% to 36%, with the rate of excellent-to-good cosmetic outcomes ranging from 66% to 89%.18 In a recently published multicentre, prospective, nonrandomised study involving 33 patients with breast cancer who underwent immediate abdominal free flap breast reconstruction after NART, not only was the safety and feasibility of NART for DIEP flap reconstruction confirmed, but there was also an even higher breast satisfaction score of 77 at 12 months postoperatively.19 We aim to conduct a randomised trial comparing QoL and oncological outcomes between preoperative radiotherapy and conventional PMRT in patients undergoing immediate deep inferior epigastric perforator (DIEP) flap breast reconstruction.
Study objectives
The aim of this study is to compare patient-reported outcomes (PROs), aesthetic outcomes and oncological safety between preoperative radiotherapy and PMRT in patients with LABC undergoing DIEP flap reconstruction.
Methods and analysis
Study design
This study is a multicentre, prospective, open-label, randomised controlled trial investigating the time of radiotherapy intervention to compare PROs and safety outcomes in patients with LABC undergoing immediate DIEP flap reconstruction.
Patients with LABC who receive neoadjuvant chemotherapy will be randomised into the following groups: (1) the preoperative radiotherapy group—preoperative radiotherapy followed by skin-sparing or nipple-sparing mastectomy combined with DIEP flap reconstruction; and (2) the postoperative radiotherapy group—skin-sparing or nipple-sparing mastectomy combined with DIEP flap reconstruction, followed by postoperative adjuvant radiotherapy.
Randomisation
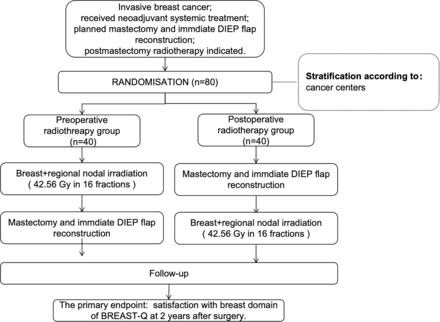
The study will employ a central randomisation scheme for participant allocation to ensure the integrity of random assignment and reduce the potential for allocation bias. The randomisation stratification factors will include the cancer centre. The study plan and treatment schedule are shown in figure 1.
Flow chart of the CAPPELLA trial. DIEP, deep inferior epigastric perforator
Study population
Only adult (>18 years old) female patients with LABC scheduled for both mastectomy and radiotherapy and deemed suitable for DIEP flap reconstruction will be included. Patients diagnosed with LABC who have primary invasive breast cancer without distant metastasis will be offered neoadjuvant therapy.
All patients will be asked to provide informed consent before enrolment and data collection. The key exclusion criteria will be stage IV breast cancer or participation in another clinical trial. Patients who experience disease progression during neoadjuvant chemotherapy will also be excluded. Patients who are pregnant or breastfeeding, who have a previous history of diabetes or who have a history of heavy smoking will also be excluded.
There will be nine centres participating in this study, and 40 consecutive patients will be recruited for each group. The study started in June 2022, expected to complete enrolment in June 2024. Data collection and statistical analyses will complete in July 2026.
Primary endpoint
The primary endpoint will be satisfaction with the breast domain of the BREAST-Q at 2 years postoperatively. The BREAST-Q is a commonly used PRO instrument for measuring QoL and patient satisfaction in breast surgery.20 The BREAST-Q questionnaire assesses patient satisfaction with their breasts, surgical outcomes, physical well-being and surgeon. It offers multiple versions of procedure-specific modules.21 22 A recent systematic review showed that the BREAST-Q can effectively be used to assess patients’ satisfaction and QoL in relation to different types of oncoplastic breast surgeries.23 This trial will use a 1:1 superiority design, assuming that the preoperative radiotherapy group will be superior to the postoperative radiotherapy group in terms of breast satisfaction.
Secondary endpoints
The secondary endpoints will include PROs at 3, 12 and 24 months postoperatively in both groups, aesthetic assessments, complication rates, rates of total pathological complete response (tpCR) and tumour safety. Prognosis will be assessed using overall survival (OS), disease-free survival (DFS) and locoregional recurrence-free survival (LRFS). DFS will be calculated as the time from breast cancer diagnosis until tumour recurrence, metastasis, contralateral second primary breast cancer or death from any cause.24 25 OS will be defined as the time from breast cancer diagnosis to the time of death from any cause or the date of last contact if death is not recorded before the cut-off date. LRFS will be defined as the time from the date of diagnosis to the recurrence of the ipsilateral chest wall, axillary, supraclavicular or internal mammary lymph node metastasis or death from any cause. A tpCR will be defined as the absence of invasive cancer in the breast and axillary lymph nodes.
Surgical complications will include haematoma, seroma, poor healing or necrotic splitting of the breast incision, nipple or areola necrosis, infection, loss of volume, unplanned reoperation, DIEP flap failure (defined as complete necrosis or partial necrosis of the flap, requiring debridement) and fat necrosis (defined as a palpable hard knot of ≥2 cm maximum diameter within the flap, with or without evidence from pathology or ultrasound).
Radiation-related complications will include radiation dermatitis, breast fibrosis, flap contracture, telangiectasia, radiation pneumonitis/pulmonary fibrosis and radiation cardiac injury.
Pictures of patients (anterior, lateral, three-fourth angled view from both sides) will be taken at baseline (before surgery) and at 3, 12 and 24 months postoperatively to assess breast aesthetics. For those who will undergo NART, photographs will be taken before NART. Changes in the photographic breast appearance will be assessed at 3, 12 and 24 months postoperatively and compared with the baseline photographs. Breast size and surgical deficit will be scored from the baseline photographs on a 3-point scale (small, medium and large). A panel of at least three independent observers blinded to patient identity, treatment allocation and radiotherapy centre will score the photographs.26 27
Radiotherapy
Timing
Preoperative radiotherapy group: Preoperative radiotherapy will be initiated 3–4 weeks following the last dose of neoadjuvant chemotherapy. Surgery will be completed within 2–6 weeks after completing radiation therapy.
Postoperative radiotherapy group: Surgery will be completed within 3–4 weeks after the last dose of neoadjuvant chemotherapy. Adjuvant radiotherapy will be initiated within 12 weeks postoperatively.
Localisation, simulation and immobilisation
Patients will undergo CT simulation in the standard supine position using breast boards or other immobilisation devices according to the treating physician’s discretion. A CT scan image thickness of ≤0.5 cm will be employed. Methods to minimise cardiac exposure, such as deep inspiration breath holding (DIBH), will be recommended for left-sided patients if available.
Target and normal tissue volume definitions
Clinical target volumes
The clinical target volumes (CTVs) will include the ipsilateral breast/chest wall, supraclavicular lymph nodes, undissected axilla and internal mammary nodes (IMNs), as defined according to the European Society for Radiotherapy and Oncology (ESTRO) and Radiation Therapy Oncology Group (RTOG) breast cancer consensus guidelines.28 Regarding controversial issues, several modifications have been made to establish an in-house consensus by the Fudan University Shanghai Cancer Centre. The suggested CTV delineation for breast and lymph node regions is given below and shown in online supplemental table 1.
Supplemental material
Breast CTV (CTV_B): CTV_B is contoured following the definitions of the ESTRO guidelines for the preoperative radiotherapy group, except for the ventral border. The ventral border of the breast CTV can be the skin or 3–5 mm under the skin surface, as determined by a multidisciplinary research team. Generally, for patients with clinical stage T4 disease and extensive skin invasion, the ventral border of the breast CTV should be the skin.
Chest wall CTV (CTV_CW): CTV_CW is also contoured following the definitions of the ESTRO guidelines for the postoperative radiotherapy group, except for the ventral border. The definition of the ventral border of CTV_CW is the same as that of CTV_B.
Undissected axilla CTV (CTV_udALN): CTV_udALN consists of the undissected portions of the axilla extending from the upper extent of axillary surgery for patients with axillary dissection (or planned axillary dissection for the preoperative radiotherapy group). CTV_udALN typically includes level 3, some of undissected axillary level 2 and interpectoral nodes. The lateral and caudal borders of CTV_udALN are defined by the most medial and cranial extent of the axillary dissection, respectively, with the other borders following the definitions of the ESTRO guidelines.
Internal mammary node CTV (CTV_IMN): CTV_IMN includes the internal mammary vessels in the first three intercostal spaces and is contoured as per the definitions of the ESTRO guidelines.
Supraclavicular lymph node CTV (CTV_SCN): CTV_SCN is contoured following the definitions of the ESTRO or RTOG guidelines. The cranial border is caudal to the cricoid cartilage; the caudal border includes the subclavian vein with a 5 mm margin and connects to the cranial border of CTVn_IMN; the medial border includes the internal jugular vein and excludes the common carotid artery and thyroid gland; the lateral border is the sternocleidomastoid muscle (cranial), clavicle and junction of the first rib (caudal); the ventral border is the sternocleidomastoid muscle and clavicle; and the dorsal border is the anterior aspect of the scalene muscle.
Planning target volumes
Planning target volumes (PTVs) will be generated by adding 5 mm margins in all directions to the above CTVs. The supraclavicular PTV will exclude the ipsilateral thyroid, trachea, oesophagus and ipsilateral lung, and the ventral border will be 3 mm beneath the skin surface. Additionally, PTV_B_evaluation and PTV_CW_evaluation will be generated by limiting the ventral border of PTV_B and PTV_CW at the skin or 3~5 mm beneath the skin surface for dose-volume histogram evaluation.
Organs at risk
Normal structures will include the heart, ipsilateral lung, contralateral lung, contralateral breast, thyroid, humeral head, spinal cord and oesophagus.
Technique and treatment planning
Intensity-modulated radiation therapy (IMRT), volumetric-modulated arc therapy (VMAT) or tomotherapy techniques will be recommended. For patients with a ventral border of the breast CTV at the skin, the application of a 3 mm bolus to the breast skin will be recommended; a 33D-printed bolus will be recommended if available. The suggested PTV dose-volume histogram criteria are as follows: at least 95% of each PTV receives ≥95% of the prescribed dose (≥90% of the prescribed dose is acceptable; for PTV_IMN, it is acceptable if ≥90% of the PTV_IMN receives ≥90% of the prescribed dose). The maximum point dose of each PTV is recommended to be ≤115% of the prescribed dose (≤120% acceptable).
Dose specifications
Patients will be treated with hypofractionated radiotherapy, and the dose regimen will be 42.56 Gy in 16 fractions. For patients with clinical stage N3c disease at initial diagnosis, a supraclavicular nodal boost may be delivered at the discretion of the treating physician. Boost doses will be 10–16 Gy in five to eight fractions if indicated.
Treatment verification
Portal films or 3D images will be obtained and approved by a physician prior to the initiation of radiotherapy and after every five fractions.
Follow-up
Before surgery, patient information, clinical and pathological characteristics, satisfaction with breast appearance and breast size measurements will be recorded. During the hospital stay, only complications and pain will be assessed. Postoperatively, at 3, 12, 24 and 36 months, the BREAST-Q score, cosmetic PROs, photographs, prognosis and long-term complications will be evaluated.
Data collection and management
Data will be recorded on the CAPPELLA case report forms (CRFs). The items collected will include patients’ demographic data (including age, sex, Body Mass Index (BMI), marital status and parental history), clinical and pathological data, surgical information, BREAST-Q score, cosmetic PROs, surgery- and radiotherapy-related complications, photographs, neoadjuvant and adjuvant treatments and prognosis. Surgery-related complications will include haematoma, seroma, poor healing, nipple or areola necrosis, infection, loss of volume, unplanned reoperation, DIEP flap failure (defined as complete or partial necrosis of the flap requiring debridement surgery) and fat necrosis. Radiotherapy-related complications will include radiation dermatitis, breast fibrosis, flap contracture, capillaritis, radiation pneumonitis/pulmonary fibrosis and radiation heart injury.
Statistical analysis and sample size calculation
Descriptive statistics will be used to summarise baseline characteristics. Proportional differences between groups will be tested using Pearson’s χ2 test. T-test will be used to compare continuous outcomes between two groups, and a one-way analysis of variance will be used to compare outcomes across groups. Univariate and multivariate logistic regression analyses will be carried out to examine associations between independent variables and outcomes. All available independent variables will be considered in the univariate regression model, and only significant variables (p<0.1) will be included for further multivariate logistic regression analyses. All tests will be two-sided, and a p value of less than or equal to 0.05 will be considered significant.
Sample size calculation
The study has been designed as a randomised, controlled superiority trial with participants allocated at a 1:1 ratio between the preoperative radiotherapy and PMRT groups. According to previous studies, the mean score of the breast domain of the BREAST-Q at 2 years postoperatively was 64±16.9 in the PMRT group.29 The average score in the breast domain of the BREAST-Q at 2 years in the preoperative radiotherapy group is estimated to reach 77 on average. We assume that the breast satisfaction score will be better in the preoperative radiotherapy group than in the PMRT group. A one-sided alpha level of 0.025 will be used for statistical significance. Thus, a sample size of 40 patients per group (80 patients in total) can provide a statistical power of 0.93 to detect significant differences between the groups, assuming equal variance in both groups. PASS 15 Power Analysis and Sample Size Software (2017) (NCSS, LLC, Kaysville, Utah, USA) was used to calculate the sample size
Patient and public involvement
Patients and the public were not involved in the design, recruitment to and conduct of study. The research question and outcome measures were not informed by patients’ priorities and preferences. The aim of this study was to evaluate patients’ experiences, including QoL, symptoms, physical and social functional abilities and psychosocial concerns. Healthcare providers and hospital staff will support this work. No patient advisers were involved in this study.
Discussion
IBR is associated with improved QoL, body image, self-esteem and confidence and has become an increasingly popular choice among patients. Radiotherapy plays an important role in the multidisciplinary treatment of breast cancer. Breast reconstruction and PMRT have become more common in the past decade. However, there is controversy surrounding the typical approach to integrating radiotherapy with IBR in breast cancer management.
Autologous reconstruction followed by radiotherapy may cause several complications, including haematoma, seroma, infection, embolism, fibrosis, fat necrosis, total or partial loss of skin flap volume and donor site problems, thereby reducing patient satisfaction and the cosmetic outcomes of breast reconstruction. Many surgeons in the UK recommend delaying reconstruction if PMRT is required.30
However, an increasing number of studies have provided favourable evidence for immediate autologous reconstruction. In 2017, a Mastectomy Reconstruction Outcomes Consortium study provided compelling data for immediate autologous breast reconstruction. This prospective, multicentre study included 175 patients who underwent immediate or delayed autologous reconstruction with PMRT, and the results showed no significant difference in the incidence of complications between the two groups after 1 year (25.9% vs 26.9%; p=0.54).31 IBR can protect women from psychosocial distress, negative body image and diminished sexual well-being when compared with delayed reconstruction.4 This evidence suggests that immediate autologous reconstruction is better tolerated with radiotherapy than does evidence from previous experience, with lower complication rates.
Over the past decade, advances in technologies have reduced the challenges in irradiated patients who have undergone immediate reconstruction. In patients who undergo breast reconstruction, the most commonly used radiotherapy techniques are IMRT and VMAT. Both techniques improve the homogeneity of the dose within the target area while reducing the radiation dose delivered to the lungs and heart. The disadvantage of IMRT and VMAT is the wide range of low-dose zones, resulting in unknown long-term adverse events.32–34
Radiotherapy techniques play a crucial role in determining the cosmetic outcome of breast reconstruction, including the use of a bolus or boost, fractionation and nodal target volumes. Orecchia et al reported dosimetric results and toxicity evaluation results of 120 patients who received hypofractionated PMRT with IMRT. Among them, 70.8% of the plans had high scores for optimal chest wall (CW) and nodal region (RN) coverage, and grade two acute toxicity was observed in 36.2% of patients.35 Dumane and colleagues reported the dosimetric results of 10 consecutive patients who underwent tissue expander/permanent implant (TE/PI) reconstruction and who were treated with a combination of VMAT and DIBH. Significant dosimetric gains were demonstrated for low doses to the heart, lungs and contralateral breast/implants.36
Although PMRT has therapeutic benefits, it can increase the risk of complications and often results in poor cosmesis for women undergoing breast reconstruction. Radiation exposure can cause early changes in normal tissues, such as skin erythema, desquamation and pruritus. These changes are caused directly by DNA damage or indirectly through the release of free radicals or inflammation. Late changes in the affected area may include damage to small blood vessels, cell loss and fibrosis. These factors can contribute to poor wound healing and potentially worse cosmetic outcomes.
Due to the limitations of current methods, there has been growing interest in using preoperative radiotherapy to avoid negative effects on autologous breast reconstruction caused by radiation. Recently, some researchers have published findings supporting preoperative radiotherapy as a proven strategy to improve cosmetic results and simplify the reconstructive process.15–17 Singh et al performed a systematic review that included 10 retrospective and eight prospective studies analysing the effects of NART on IBR; the overall complication rates ranged from 3% to 36%, and the rates of excellent-to-good cosmetic outcomes ranged from 66% to 89%.18 However, there are limited data on the effect of preoperative radiotherapy prior to mastectomy and microvascular autologous reconstruction.37 The PRADA study recently demonstrated that preoperative radiotherapy followed by skin-sparing mastectomy and DIEP flap reconstruction is technically feasible. The study reported low rates of surgical complications and good short-term oncological outcomes.19 These studies lack longer follow-up evaluations, and larger prospective controlled clinical trials are required to objectively measure this new therapeutic sequence. We aim to compare the oncological and QoL outcomes in a randomised trial of preoperative radiotherapy versus conventional PMRT in breast reconstruction.
Ethics and dissemination
This study will be performed in accordance with the Helsinki Declaration. Patients will be provided with details of the study (purpose, risk and benefits) and have the right to withdraw from the study at any time. Approval for this study was provided by the independent ethics committee and institutional review board of Fudan University Shanghai Cancer Centre and approval ID number was 2208258-5-2402C. Prior to enrolment, an informed consent form will be sent to each patient to ensure their understanding of the cohort study. This study will be conducted according to the requirements of the Good Clinical Practice guidelines. The results of the study will be presented at national and international meetings and published in a scientific peer-reviewed journal.
Ethics statements
Patient consent for publication
References
Footnotes
SH, JH and LZ are joint first authors.
Contributors Authors’ contributions Study design: JW, XY, SH, LZ and CZ Protocol writing: SH, JH and LZ. Protocol review: JW, XY, YH, KY, ZH, GL, GD, ZS Guarantor is JW.
Funding This research is supported by the Shanghai Science and Technology Commission (22DX1900500)
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer-review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.