Article Text
Abstract
Introduction Graft-versus-host disease (GvHD) remains a major complication of allogeneic stem cell transplantation (allo-SCT), affecting 30–70% of patients (representing 800 new patients per year in the UK). The risk is higher in patients undergoing unrelated allo-SCT. About 1 in 10 patients die as a result of GvHD or through complications of its treatment. In patients who survive GvHD and or the complications of treatment, about 1 in 3 patients develop chronic GvHD which has a negative impact on quality of life (QoL). Many transplant protocols use alemtuzumab or anti-thymocyte globulin (ATG) in combination with a calcineurin inhibitor (CNI) and mycophenolate mofetil as GvHD prophylaxis; however, the outcomes of these treatments are complicated by high rates of infection and therefore the development of improved GvHD prophylaxis strategies represents a major unmet need.
Methods and analysis The Methods of T-Cell Depletion trial is a prospective, multicentre, adaptive randomised trial in patients undergoing reduced intensity-conditioned unrelated donor allo-SCT. The trial will compare the novel GvHD prophylaxis regimens post-transplant cyclophosphamide (PTCy) in combination with a CNI or sirolimus (PTCy-CNI or PTCy-sirolimus, respectively) to a current standard-of-care GvHD prophylaxis involving the use of Thymoglobulin (a specific brand of ATG containing rabbit polyclonal antibodies). The primary outcome measure is GvHD-free, relapse-free survival at 1 year. Secondary outcomes include cumulative incidence of acute grade II-IV GvHD at 1 year, cumulative incidence of moderate and severe chronic GvHD at 1 year, overall survival at 1 year and cumulative incidence of non-relapse mortality at 1 year.
Ethics and dissemination The protocol was approved by the West Midlands, Edgbaston Research Ethics Committee (20/WM/0195); initial approval was received on 11 September 2020, current protocol version (V.4.0) approval on 25 July 2023. The Medicines and Healthcare products Regulatory Authority also approved all protocol versions. The results of this trial will be disseminated through national and international presentations and peer-reviewed publications.
Trial registration number EudraCT Number: 2019-002419-24.
ISRCTN Number: 50290131.
- Patients
- Clinical Trial
- Bone marrow transplantation
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Adaptive trial design including a ‘pick a winner’ format provides an efficient use of resource.
The composite primary clinical endpoint of graft-versus-host disease (GvHD)-free, relapse-free survival is commonly used by other randomised clinical trials of GvHD prevention and measures long-term morbidity as well as survival.
Trial participants will provide blood samples for detailed analysis of immune reconstitution.
Trial recruitment will benefit from being part of the UK IMPACT (UK partnership for transplant trials) portfolio.
Patient population is heterogeneous, necessitating stratification for parameters predicted to impact outcome.
Introduction
Graft-versus-host disease (GvHD) remains a major complication of allogeneic stem cell transplantation (allo-SCT) affecting 30–70% of patients (representing about 800 new patients per year in the UK). The risk is higher in patients undergoing unrelated donor allo-SCT.1 2 About 1 in 10 patients die as a result of GvHD or complications of its treatment (eg, infection). In surviving patients, about 1 in 3 patients develop chronic GvHD (cGvHD) which has a negative impact on quality-of-life.1 2 In patients undergoing reduced intensity conditioned (RIC) unrelated donor allo-SCT, many UK and European transplant centres use alemtuzumab or anti-thymocyte globulin (ATG) in combination with a calcineurin inhibitor (CNI) and mycophenolate mofetil (MMF) as GvHD prophylaxis. The use of alemtuzumab or ATG delivers profound in vivo lymphocyte depletion and has been shown to reduce the risk of acute and chronic GvHD in several clinical trials,3–5 although one study showed inferior survival.6 In vivo serotherapy to induce lymphocyte depletion results in delayed immune reconstitution post-transplant, which may increase the risk of serious infection and a possible increased risk of relapse. The development of improved GvHD prophylaxis strategies therefore represents a major unmet need.
Recently the administration of high-dose cyclophosphamide 3–4 days following transplant of an un-manipulated peripheral blood stem cell grafts from human leucocyte antigen (HLA)-matched donors has been shown to prevent GvHD. Post-transplant cyclophosphamide (PTCy) leads to impaired proliferation of alloreactive CD4+ and CD8+ T cells in vivo.7 It has been hypothesised that the remaining T-cell repertoire (including pathogen-specific T cells) is less affected leading to quicker immune reconstitution and less infection.8 Originally evaluated in the context of haploidentical transplantation, recent studies have addressed PTCy utility following HLA-matched transplantation. When compared with CNI-based GvHD prevention strategies for RIC transplants without T-cell depletion and involving peripheral blood stem cells from HLA-matched donors, two recent prospective trials (HOVON-96 and BMT CTN 1703) have demonstrated that PTCy-CNI improved 1-year GvHD-free, relapse-free survival (GRFS)9 10; these data confirmed the superiority compared with non-lymphocyte depleting GvHD prophylaxis protocols, as suggested by a prior prospective study (BMT CTN 1203) in which the PTCy-CNI experimental group was compared with a contemporary control arm.11 When combined with myeloablative conditioning and transplantation of bone marrow, PTCy without additional CNI is associated with similar outcomes to standard T-replete controls using standard CNI-based GvHD prophylaxis.12 However, it is unclear whether PTCy can improve clinical outcomes when compared with strategies that involve other manoeuvres to deplete lymphocytes from the graft. While PTCy alone is insufficient to prevent GvHD following unrelated peripheral blood stem cell donor transplant,8 it is also unclear which other immunoprophylactic drugs will be best in combination. For example, in the mismatched unrelated donor setting, sirolimus (a mammalian target of rapamycin inhibitor) has been deployed rather than a CNI.13 The different immune effects of each drug and their distinct toxicities have the potential to affect the overall efficacy of the PTCy. This randomised trial will therefore test two novel methods of GvHD prevention involving the use of PTCy (combined with MMF and either a CNI or sirolimus) and compare these to a control involving a current standard-of-care, Thymoglobulin (a specific brand of ATG containing polyclonal rabbit antibodies) with a CNI and MMF. A key question therefore is whether either of these PTCy platforms is superior to in vivo lymphocyte depletion with Thymoglobulin.
Methods and analysis
Study design
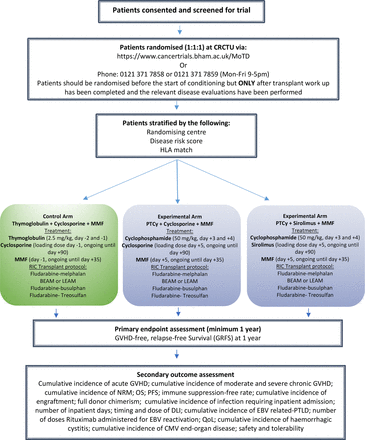
The Methods of T cell Depletion (MoTD) trial is a multicentre, prospective phase II, adaptive, randomised clinical trial in patients undergoing RIC unrelated peripheral blood stem cell donor allo-SCT. The trial will compare the novel GvHD prophylaxis regimens of PTCy-CNI or PTCy-sirolimus to a current standard-of-care involving the use of T-cell depletion with Thymoglobulin, in combination with a CNI (figure 1 (trial schema)). The trial will recruit 400 patients across the treatment arms, randomisation will be performed according to a 1:1:1 ratio (control arm vs experimental treatment arms) using a minimisation procedure to stratify based on randomising centre, disease risk score (low/intermediate vs high/very high) and HLA match (10/10 vs 9/10).
Trial schema. Beam - carmustine, etoposide, cytarabine and melphalan; CMV - cytomegalovirus;DLI - donor lymphocyte infusion; EBV, Epstein-Barr virus; GVHD, graft-versus-host disease; HLA, human leucocyte antigen; Leam - carmustine, lomustine, etoposide and cytarabine; MMF, mycophenolate mofetil; NRM - non relapse mortality; OS - overall survival; PFS - Progression free survival; PTCy, post-transplant cyclophosphamide; PTLD - post transplant lymphoproliferative disorder; QoL, quality of life; RIC, reduced intensity conditioned.
Patient and public involvement
From its inception, the MoTD trial was co-developed with the patient and public involvement group based in the Cancer Research UK Clinical Trials Unit at the University of Birmingham and a Patient Group from Anthony Nolan. The group refined the protocol and participant-facing documents and provided input into the design.
Inclusion and exclusion criteria
Patients who have a suitably matched unrelated donor (9/10 or 10/10); planned to receive one of the following RIC protocols- fludarabine-melphalan (fludarabine 120–180 mg/m2 intravenous; melphalan <150 mg/m2 intravenous), BEAM (carmustine, etoposide, cytarabine and melphalan) or LEAM (carmustine, Lomustine, Etoposide and cytarabine) (carmustine 300 mg/m2 intravenous or lomustine 200 mg/m2 intravenous with: etoposide 800 mg/m2 intravenous; cytarabine 1600 mg/m2 intravenous; melphalan 140 mg/m2 intravenous), fludarabine-busulphan (fludarabine 120–180 mg/m2 intravenous; busulphan <8 mg/kg PO or <6.4 mg/m2), fludarabine-treosulfan (fludarabine 150 mg/m2 intravenous; treosulfan 30 g/m2 intravenous), planned use of peripheral blood stem cells for transplantation; planned allo-SCT for one of the following-acute myeloid leukaemia in complete remission (CR), acute lymphoblastic leukaemia (ALL) in CR, chronic myelomonocytic leukaemia <10% blasts, myelodysplastic syndromes <10% blasts, non-Hodgkin’s lymphoma in CR/partial response (PR), Hodgkin’s lymphoma in CR/PR, multiple myeloma in CR/PR, chronic lymphocytic leukaemia in CR/PR, chronic myeloid leukaemia in first or second chronic phase and myelofibrosis; patients aged 16–70 years; females and male patients of reproductive potential must agree to use appropriate, highly effective, contraception from the point of commencing therapy until 12 months after transplant. Exclusion criteria include use of any method of graft manipulation, use of alemtuzumab or any method of T-cell depletion except those that are protocol-defined, known hypersensitivity to study drugs or history of hypersensitivity to rabbits, pregnant or lactating women, adults of reproductive potential not willing to use appropriate, highly effective, contraception during the specified period, life expectancy <8 weeks, active hepatitis virus or hepatitis C virus infection, organ dysfunction (defined as left ventricular ejection fraction <45%, glomerular filtration rate <50 mL/min, bilirubin >50 µmol/L, aspartate transaminase or alanine transferase >3 × upper limit of normal), participation in the COSI (ISRCTN Number: 12434060) or ALL-RIC (ISRCTN Number: 99927695) trials any contraindication to treatment with the study drugs (Thymoglobulin, cyclophosphamide, sirolimus, cyclosporine and mycophenolate mofetil) as detailed in each study drug summary of product characteristics (SmPCs) and patient has any other systemic dysfunction or significant disorder which, in the opinion of the investigator would jeopardise the safety of the patient by taking part in the trial.
Consent
Patients are identified as per-site established processes. Each eligible patient will be given a patient information sheet (PIS) by the site investigator who will ensure that they adequately explain the aim, trial treatment, anticipated benefits and potential hazards of taking part in the trial to the patient. The right of the patient to refuse to participate in the trial without giving a reason will be respected. Written informed consent is requested from the patient by the investigator who has been delegated the responsibility on the delegation log.
Interventions
Control arm (Thymoglobulin + cyclosporine + MMF)
Thymoglobulin is given as an intravenous infusion of 2.5 mg/kg/day over 2 days (days −2 and −1; total dose 5 mg/kg) via a central line through a 0.2 micron inline filter. Each dose will be infused over 6–8 hours. No test dose will be given. 30 min before Thymoglobulin, the patient should receive methylprednisolone 1 mg/kg intravenously, 1 g paracetamol PO (by mouth) and 10 mg chlorphenamine intravenous. Patients should be monitored carefully and receive appropriate therapy for any infusion-related or anaphylactic reactions as per local policy.
Patients will receive intravenous/PO cyclosporine according to local policy to begin on day −1 maintaining a trough level of 100–200 µg/L until day 90 before a subsequent taper in the absence of any active GvHD.
MMF will be given intravenous/PO according to local policy at a dose of 1 g three times a day to begin on day −1 and discontinued on day 35 without taper if there is no evidence of active GvHD. In adults weighing <55 kg, MMF should be given at a lower dose of 0.75 g intravenous/PO three times a day.
Experimental arm (PTCy + cyclosporine + MMF)
Cyclophosphamide is given as an intravenous infusion of 50 mg/kg/day over 2 days (days 3 and 4; total dose 100 mg/kg) together with intravenous hydration and mesna, as per local policy.
Where possible cyclophosphamide should be given exactly at 72 hours and 96 hours after the stem cell infusion, however, we realise logistical issues can make this difficult. On this basis, centres should define day+3 and day+4 according to a 24-hour time window for each day and ensure that the drug is given within these windows. For example, day+3 cyclophosphamide could be given at 60–84 hours and day+4 cyclophosphamide given 84–108 hours, ensuring a 24-hour interval between doses.
Patients will receive intravenous/PO cyclosporine according to local policy to begin on day 5 maintaining a trough level of 100–200 µg/L until day 90 before a subsequent taper in the absence of active GvHD.
MMF will be given intravenous/PO according to local policy at a dose of 1 g three times a day to begin on day 5 and discontinued on day 35 without taper if there is no evidence of active GvHD. In adults weighing <55 kg, MMF should be given at a lower dose of 0.75 g intravenous/PO three times a day.
Experimental arm (PTCy + sirolimus + MMF)
Cyclophosphamide is given as an intravenous infusion of 50 mg/kg/day over 2 days (days 3 and 4; total dose 100 mg/kg) together with intravenous hydration and mesna, as per local policy.
Sirolimus will be initially given PO as a loading dose of 6 mg on day 5 followed by 2 mg daily; doses will be adjusted to maintain a trough level (in whole blood) of 8–14 ng/mL until day 60, thereafter 5–8 ng/mL until day 90. In the absence of active GvHD, the dose of sirolimus will be tapered from day 90. We strongly recommend pre-emptive reductions of the loading dose and daily maintenance dose of sirolimus see table 1, when there is concomitant treatment with a triazole anti-fungal agent. Drug levels should be monitored closely on introduction or cessation of triazole drugs.
Sirolimus dose reduction with concomitant treatment use of triazole anti-fungal agent
Unlike when letermovir is co-administered with cyclosporine, when a lower dose of letermovir (240 mg) is used, when co-administered with sirolimus the full dose of letermovir 480 mg once a day should be prescribed.
MMF will be given intravenous/PO according to local policy at a dose of 1 g three times a day to begin on day 5 and discontinued on day 35 without taper if there is no evidence of active GvHD. In adults weighing <55 kg, MMF should be given at a lower dose of 0.75g intravenous/PO three times a day.
Dose modifications other than those already mentioned are not permitted in this trial unless they are in line with the SmPC. Whole blood levels of sirolimus and cyclosporine should be closely monitored in patients with hepatic impairment and when inducers or inhibitors of CYP3A4 (and P-glycoprotein for cyclosporine) are concurrently administered. For dose adjustments, maybe necessary, refer to the relevant section of the sirolimus and cyclosporine SmPC and online supplemental appendix 1.
Supplemental material
If a patient discontinues sirolimus or cyclosporine before the planned taper and discontinuation, MMF should be continued or restarted as GvHD prophylaxis. Tacrolimus should not be used to substitute for either drug.
Supportive treatment is permitted for patients and is detailed in online supplemental appendix 2. Concomitant medication may be given as medically indicated, unless otherwise specified in the SmPC for the (Investigational Medicinal Product) IMPs. Online supplemental appendix 1 provides guidance on medications with potential interactions with the IMPs.
Supplemental material
In addition to routine post-transplant visits all patients will be formally reviewed at day+100 and then, 6, 9 and 12 months. Other assessments include monitoring for EBV reactivation as per local policy and reported in the event of a reactivation. Disease assessment will be performed according to local policy. Assessment will be performed at baseline and then as per local practice post-transplant (eg, at day 90 and months 6, 9 and 12 post-transplant). GvHD (acute and chronic) will be assessed continually until the end of the trial; weekly for the first month post-transplant (day 7, day 14, day 21, day 28), day 100 and months 6, 9 and 12 post-transplant. aGvHD (acute Graft Versus host disease) will be assessed using the modified Glucksberg criteria and cGvHD will be assessed using the National Institutes of Health criteria. Engraftment will be assessed by lineage-specific chimerism measurements performed at 3 monthly intervals for the first 12 months post-transplant; at day 100 and then months 6, 9 and 12. QoL will be assessed using the FACT-BMT (The Functional Assessment of Cancer Therapy - Bone Marrow Transplantation (questionnaire at baseline and months 6 and 12 post-transplant. Collection of lymphocyte subsets—numbers of CD3, CD4, CD8, CD19 and CD56 cells should be collected at day 100 and months 6, 9 and 12.
The planned start date of the study is 01 January 2019 and the end date is 01 January 2026. The recruitment start date is 22 February 2021 and the end of recruitment is 30 January 2025.
Trial outcomes
The primary outcome is GvHD-free, relapse-free survival at 1 year. The secondary outcomes are: (1) cumulative incidence of acute grade II-IV and III-IV GvHD at 1 year; (2) cumulative incidence of moderate and severe chronic GvHD at 1 year; (3) cumulative incidence of non-relapse mortality at 1 year; (4) overall survival at 1 year; (5) progression-free survival at 1 year; (6) immune suppression-free survival at 1 year; (7) cumulative incidence of engraftment at 1 year; (8) the incidence of full donor chimerism at 100 days; (9) cumulative incidence of infection requiring inpatient admission at 1 year; (10) the number of inpatient days during first 12 months; (11) the timing and dose of donor lymphocyte infusion for mixed chimerism, persistent disease or relapse; (12) cumulative incidence of EBV-related post-transplant lymphoproliferative disorder; (13) the number of doses of rituximab administration for EBV reactivation during first 12 months; (14) QoL measured by FACT-BMT questionnaire at baseline, 6 and 12 months; (15) cumulative incidence of patients with haemorrhagic cystitis at 1 year; (16) cumulative incidence of cytomegalovirus end-organ disease at 1 year; (17) safety defined as incidence of ≥grade 3 toxicities reported as per National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) V.4.0.; and (18) tolerability defined to be a number of patients able to complete therapy as scheduled. Additional exploratory biological endpoints relating to immune reconstitution and GvHD biomarker evaluation will also be performed.
Statistical analysis plan
A stage 1 interim analysis, based on the incidence of acute GvHD (grade III-IV), will be performed when there is complete data at 100 days for 150 patients (50 per treatment). If there is a probability of greater than 0.9 that an experimental arm is inferior to the control arm, then the experimental arm will be dropped. Arms that are substantially worse than control (20% incidence vs 10% or 30% vs 20%) have a high probability of being stopped at this interim analysis (approximately 43–54%). However, arms that are slightly better (5% vs 10% or 15% vs 20%) have a very low probability of stopping (1–2%).
A stage 2 interim analysis will be performed after 300 patients have been transplanted; this will evaluate the primary outcome, GRFS. Here the options are: (1) early conclusion of the trial, if both experimental arms have a high probability of superiority to the standard of care; (2) early termination, if both arms are clearly inferior to the standard of care; (3) dropping of one experimental arm (4) continued randomisation between the two intervention and control arms up to 400 patients. If both intervention arms are superior to control, there will be approximately 33% probability of early conclusion for intervention superiority if the HR for each intervention arm relative to control is 0.7, and 13% probability if the HRs are 0.8.
For the final analysis we define a criterion for ‘success’ of the trial, to allow approximate comparison with a traditional frequentist design; a probability of >95% of benefit to at least one intervention arm at the final analysis, or early stopping (at the second interim analysis) for benefit. The probability of success (by this definition) is approximately 78% if there is one experimental arm with an HR of 0.7, or 89% if both arms have an HR relative to standard care of 0.7. For comparison, a traditional fixed trial would require a sample size of about 350 for a two-group comparison to detect an HR of 0.7 with 80% power and one-sided type 1 error rate of 5%, therefore 525 would be required for a three-armed trial.
The main analysis of the primary and secondary outcome measures will be performed once all randomised patients have been transplanted followed-up for a minimum of 12 months, and data collection is complete. The primary outcome measure of GRFS is defined as the time from the date of day 0 (defined as the day of stem cell infusion) to the date of the first event or death from any cause. An event is defined as GvHD (acute grade III-IV and/or chronic GvHD requiring systemic immune suppressive treatment), disease relapse or progression. Patients who are alive and event-free at the end of the trial will be censored at their date of last follow-up. Bayesian statistical methods for the interim and final analyses will be used. GRFS will be modelled using appropriate survival analysis techniques taking into consideration the use of parametric models, with adjustment for stratification variables and other important prognostic factors. Posterior distributions for treatment effects (and other parameters of interest) will be estimated and displayed graphically summarised by a point estimate of the HR, with uncertainty shown by the 95% highest density intervals.
Adverse events reporting and analysis
The collection and reporting of adverse events (AEs) will be in accordance with the Medicines for Human Use Clinical Trials Regulations 2004 and its subsequent amendments. The reporting period for AEs will be from the date of commencement of protocol-defined treatment until 28 days after the administration of the last dose of IMP. Serious (S)AEs will be reported from the date of consent. Adverse events of special interest (AESI) are ones of scientific and medical interest specific to the understanding of the IMP and may require close monitoring. These should be reported as an SAE and irrespective of how long after IMP administration the reaction occurred and should be reported for ALL study arms. The following AESI’s using the CTCAE V.4.0 terminology and grading with a grade 2 are of interest; arrhythmia, other ECG abnormality, myocarditis, pericarditis, pericardial effusion, heart failure and/or reduced left ventricular systolic function, myocardial infarction and ischaemia. AESI should not be reported if they were present at baseline unless they have increased in severity. The investigator should assess the seriousness and causality (relatedness) of all AEs experienced by the patient (this should be documented in the source data) with reference to the SmPCs. Abnormal laboratory findings will only be reported if they (1) result in early discontinuation from the study treatment; (2) require study drug dose modification or interruption, any other therapeutic intervention or is judged to be of significant clinical importance. Pre-existing conditions should only be reported if the condition worsens by at least one CTCAE grade. Hospitalisations for protocol-defined treatment (including admission for the transplant) or preplanned elective procedures unless the condition worsens will not be reported as SAEs.
Data management
Data will be collected via a set of forms capturing details of eligibility, baseline characteristics, treatment and outcome details. This trial will use an electronic remote data capturing system, with the exception of SAE reporting and pregnancy notification, both of which will be paper-based. All trial records must be archived and securely retained for at least 25 years. No documents will be destroyed without prior approval from the sponsor, via the central MoTD Trial Office. On-site monitoring will be carried out as required following a risk assessment and as documented in the Quality Management Plan. Any monitoring activities will be reported to the MoTD Trial Office and any issues noted will be followed-up to resolution. MoTD will also be centrally monitored, which may trigger additional on-site monitoring. Further information regarding data management is provided in the study protocol.
Trial organisation structure
The University of Birmingham will act as a single sponsor for this multicentre study. The trial is being conducted under the auspices of the Cancer Research UK Clinical Trials Unit (CRCTU), University of Birmingham. The Trial Management Group (TMG) is responsible for the day-to-day running and management of the trial; members include the chief investigator, deputy chief investigator, co-investigators, trial statisticians, trial management team leader and trial coordinator. The TMG reports to the Trial Steering Committee (TSC). The TSC provides oversight and governance; members include independent clinicians, members of the TMG and the CRCTU trial management team leader. Other members/observers may be invited if appropriate. The TSC supervises the conduct of the trial monitoring progress including recruitment, data completeness and deviations from the protocol. They will make recommendations about the conduct and continuation of the trial. The independent Data Monitoring Committee (DMC) includes clinicians and a statistician who will review unblinded data analyses to advise the TSC on whether trial data justified the continuing recruitment of further patients. The DMC will operate in accordance with a trial-specific charter based on the template created by the Damocles Group. During the recruitment phase of the trial the DMC is scheduled to meet once 10 patients have been treated on the experimental arm to review early safety data. Subsequent meetings will be held annually thereafter. These may occur more frequently if the DMC deem necessary due to the speed of recruitment or safety issues. The funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data or decision to submit results.
Confidentiality statement
Confidential trial data will be stored in accordance with the General Data Protection Regulation 2018. As specified in the patient information sheet and with the patient’s consent, patients will be identified using only their date of birth and unique trial ID number.
Trial status
Recruitment for the trial opened 0n 22 February 2021 and is due to close to recruitment on 30 January 2025. The first patient was recruited on 14 April 2021.
Discussion
Unrelated peripheral blood stem cell allo-SCT remains the most common form of allogeneic transplantation performed worldwide. While improvements in patient and donor selection, the use of RIC protocols and better supportive care have improved clinical outcomes, infections and GvHD continue to represent major barriers to treatment efficacy.14 There remains substantial uncertainty regarding the best approach to prevent GvHD while avoiding an excessive rate of infection and relapse. Recent data from prospective randomised controlled trials shows that there are likely better methods to prevent GvHD than standard GvHD prophylaxis using T-replete protocols (mostly referring to the combination of a CNI and methotrexate or MMF).3 5 9 10 15 The MoTD trial seeks to determine how two broad strategies, both showing superiority in randomised controlled trials to standard prophylaxis, will compare. Our choice of the control arm dose schedule was based on the outcome of the Canadian randomised controlled trial5 which showed greater efficacy of low-dose Thymoglobulin+calcineurin inhibitor+MMF or methotrexate to standard GvHD prophylaxis following RIC unrelated allo-SCT; the patient population in this study conformed most closely to the eligible patient population for the current trial. The PTCy experimental arms included either cyclosporine+MMF9 11 or sirolimus-MMF,13 as these aligned with published trial protocols available at the time our trial was designed. Recent data have raised the possibility that lower doses of PTCy can be effective at preventing GvHD16 and interpretation of the results of this trial will need to be viewed in the context of the future evolution of the PTCy platform.
In deciding our primary endpoint, we have used the same composite of % 1-year GRFS that was used in recent randomised controlled trials; this is important in aiding interpretation of the study results in comparison to other, independent trials. The adaptive design also permits a ‘pick a winner’ approach with the potential to allow change in the trial arm composition after planned interim analyses; this is the most efficient way to deploy resources, avoiding the need to perform separate trials. Although the study population is not uniform in terms of blood cancer diagnosis, we have sought to mitigate against bias by employing stratification using a validated disease risk score.17
The toxicity profiles of the investigational medicinal products are distinct and our study includes prospective capture of particular outcomes (eg, EBV reactivation or haemorrhagic cystitis) that we hypothesise will differ between the groups. Emerging data also indicate that each modality may have different effects on other transplant outcomes, such as time to engraftment9 and these data will also be collected. By collecting blood samples at timed intervals from participants, we will be in a position to measure how immune recovery and GvHD biomarkers compared between the different trial arms and to define the overall intensity of immune suppression. These samples will also be stored for future ethically-approved research. As the trial continues to recruit, we continue to monitor the literature and other resources to assess ongoing trial safety. Although patients with significantly impaired left ventricular effector function are excluded from participation, a recent retrospective study suggested that patients receiving transplant and PTCy had a higher incidence of acute cardiac events than other methods of prophylaxis.18 While none of the prospective randomised controlled studies involving a PTCy experimental arm identified a higher risk of cardiac events,8 9 11 as a precautionary measure, we have modified the V.4.0 trial protocol to mandate reporting of defined cardiac events >2 CTCAE V.4.0 across all study arms as an adverse event of special interest. Together with other published prospective data, this will be crucial in defining the safety profile of each modality.
The outcome of the study may provide data to support a new standard-of-care for GvHD prophylaxis or indicate that other experimental approaches should be tested. This knowledge may be used to define the control arm for future trials and to provide information to inform the relevant trial design. Recent experience from other settings (eg, COVID-1919), suggest that adaptive trials can be run successfully as platform trials, with no defined end date, allowing for testing multiple interventions with the addition of new arms or discontinuation of treatment arms according to the emerging data. While this may provide the most efficient use of resources, the challenge will be to identify a funding model that can sustain this approach.
Ethics and dissemination
The trial will be performed in accordance with the recommendations guiding physicians in biomedical research involving human subjects, adopted by the 18th World Medical Association General Assembly. Helsinki, Finland and stated in the respective participating countries laws governing human research, and Good Clinical Practice. The initial protocol was approved by the West Midlands- Edgbaston Research Ethics Committee (REC Ref: 20/WM/0195) on 11 September 2020 with subsequent amendments approved on the 20 October 2020 (change of PI (Principle Investigator), addition of exclusion criteria, additional information on pregnancy testing and removal of the use of the GvHD application), 29 October 2020 (change of PI), 18 November 2020 (addition of new site), 24 November 2020 (change of PI), 07 December 2020 (change of two PIs and addition of new PI), 07 January 2021 (change of PI), 22 March 2021 (change of PI), 19 July 2021 (addition of fludarabine-tresosulfan as a conditioning regimen, reference to particular disease assessments removed, inclusion of remote consenting and clarification of dosing and monitoring sirolimus), 25 July 2023 (addition of reporting AESI’s linked to cyclophosphamide related to cardiac toxicity events, clarification of treatment schedule for cyclophosphamide, clarification of dosing of overweight patients and clarification of trial patients being permitted to continue on to the RATinG trial). The Medicines and Healthcare products Regulatory Authority has given its approval of all protocol versions, the current version in use is 4.0. Results of this trial will be submitted for publication in a peer-reviewed journal. The manuscript will be prepared by the TMG and authorship will be determined by mutual agreement and according to the publication policy of IMPACT (UK partnership for transplnat trials) and the CRCTU. The results will also be made available on public websites.
Ethics statements
Patient consent for publication
Acknowledgments
We thank staff from the CRCTU, University of Birmingham
References
Footnotes
Contributors Study conception: RC (Oxford Cancer and Haematology Centre, The Churchill Hospital, Old road, Headington, Oxford, OX3 7LE, UK), EK (Department of Haematology, University Hospital of Wales, Cardiff, CF14 4XW, UK), FD (Department of Clinical Haematology, Manchester Royal Infirmary, Manchester, Oxford Road, M13 9WL, UK), AB (Department of Haematology, The Christie NHS Foundation, Manchester, M20 4BX, UK), MC (Department of Haematology, Newcastle Freeman Hospital, Freeman Road, High Heaton, Newcastle upon Tyne, NE7 7DN, UK), KR (Department of Haematology, University College London Hospitals NHS Foundation, Euston Road, London NW1 2BU, UK), JD (Centre for Haemato-Oncology, Barts Cancer Institute, Charterhouse Square, London, EC1M 6AU, UK), VP (Department of Haematology, King’s College London, Strand, London, WC2R 2LS, UK), RM (Department of Haematology, Addenbrookes, Hills Road, Cambridge, CB2 0QQ), SS (Pharmacy Department, University Hospital of Wales, Cardiff, CF14 4XW, UK), SG (Cancer Research UK Clinical Trials Unit, Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, B15 2TT, UK), CG (Cancer Research UK Clinical Trials Unit, Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, B15 2TT, UK), RB (Cancer Research UK Clinical Trials Unit, Centre for Clinical Haematology, Queen Elizabeth Hospital, Bimingham, B15 2TH, UK), GS (Cancer Research UK Clinical Trials Unit, Centre for Clinical Haematology, Queen Elizabeth Hospital, Bimingham, B15 2TH, UK), AH (Cancer Research UK Clinical Trials Unit, Centre for Clinical Haematology, Queen Elizabeth Hospital, Bimingham, B15 2TH, UK). Study design: RC, EK, FD, AB, MC, KR, JD, VP, RM, SS, SG, CG, RB, GS, AH. CG is the Senior Trial Biostatistician who was responsible for developing the statistical plan. RC, EK, CG and AH were involved in all aspects of the manuscript: the concept of the manuscript, drafting the manuscript, editing the manuscript and approving the final version. These four authors are accountable for all aspects of this work. All other authors were involved in editing and approving the manuscript, FD, AB, MC, KR, JD, VP, RM, SS, SG, RB and GS. RC is the guarantor.
Funding This is a clinician-initiated and clinician-led trial funded by IMPACT. The IMPACT funders are Anthony Nolan, LEUKA and NHS Blood & Transplant. No individual per patient payment will be made to NHS trusts, Investigators or patients. The trial has been adopted into the NIHR CRN portfolio adoption.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.