Article Text
Abstract
Objectives Clozapine is continuously underused. The existing systematic reviews addressing barriers to clozapine prescribing primarily focus on clinical staff’s attitudes and perceived barriers to prescribing. However, a preliminary literature search revealed additional literature on the subject not previously included in systematic reviews, including literature on patient perspectives. A scoping review is warranted to map the scope of primary studies on patients’ and/or clinicians’ perspectives on clozapine treatment and to identify gaps in research.
Design A scoping review was designed and reported in accordance with established guidelines for scoping reviews.
Data sources The electronic databases Cochrane Library, CINAHL, Web of Science, PsycINFO, MEDLINE, EMBASE, Google Scholar and two grey literature databases were searched. Furthermore, citation tracking of selected studies was undertaken.
Eligibility criteria We included primary, empirical studies reporting clinicians’ and/or patients’ perspectives on clozapine treatment. No limitation was set for the year of publication or type of primary study.
Data extraction and synthesis Two researchers independently screened for studies, extracted the data and coded the content. Findings were summarised visually and narratively.
Results 146 studies were included. Most studies reported on patients’ or clinicians’ perspectives on active clozapine treatment or on clinicians’ perspectives on barriers to clozapine initiation in general. Three gaps in research were identified: (1) studies exploring clozapine-eligible, yet clozapine-naïve, patients’ attitudes towards clozapine commencement, (2) studies exploring clinicians’ reasons for clozapine withholding and perceived facilitators of clozapine treatment in individual patient-cases and (3) studies on patient and clinician perspectives on clozapine discontinuation, continuation and rechallenge in individual patient cases.
Conclusions Research on clozapine perspectives tends to repeat itself. Future studies addressing the identified gaps in evidence could provide the insights needed to optimise clozapine utilisation.
- Review
- PSYCHIATRY
- CLINICAL PHARMACOLOGY
- Schizophrenia & psychotic disorders
- Attitude
- NEUROLOGY
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. The data supporting the findings of this study are retrievable from the data repository The Open Science Framework (OSF) at https://doi-org.ezproxy.u-pec.fr/10.17605/OSF.IO/8T5PV in the file 'Individual study data'196 or available as supplementary materials attached to the article.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The prospective registration and publication of the review protocol has ensured transparency of the review process.
The search strategy has ensured a comprehensive search of the literature and multiple booster searches on Google Scholar have ensured a continued update on the scope of literature, the most recent one in January 2024.
The original literature search was conducted in June 2021.
The search was restricted to publications in the English language, which may have precluded the identification of some relevant insights and studies.
Introduction
Schizophrenia is a serious mental illness with major societal and personal costs.1 Early, adequate treatment is crucial to improve the long-term outcome.2 3 However, some patients with schizophrenia experience insufficient treatment response to conventional antipsychotics (APs) even if these are trialled adequately (as defined by guidelines4) and from different groups of APs. Patients with poor response to at least two different APs trialled adequately are considered treatment-resistant.4 Approximately one-third of all patients with schizophrenia are treatment-resistant, and some will develop treatment resistance over time.5
The atypical AP clozapine has shown superior to other APs in terms of overall symptom reduction for schizophrenia and related disorders6 and most guidelines recommend that clozapine should be offered first-line to patients with treatment-resistant schizophrenia,4 5 7 in which case approximately two-thirds (40%–80%) of the patients are likely to respond.8–10
It is furthermore indicated for a range of other disorders dependent on national guidelines,7 for example, schizophrenia or schizoaffective disorder with recurrent suicidality, treatment-resistant schizoaffective or bipolar disorder, psychotic disorders with poor tolerance to conventional neuroleptics, treatment-resistant organic psychosis, etc.
Regardless of the recommendations and established advantages of clozapine treatment, clozapine is underused in most parts of the world,11–16 as it has been for decades, despite a steady flow of studies on the subject.17
The clozapine underutilisation represents a major mental health concern and a few systematic reviews18–22 have aimed to summarise the identified barriers and facilitators of clozapine prescribing. These previous reviews tend to include the same studies, primarily surveys of clinical staff’s attitudes and perceived barriers to prescribing, and until recently, only a few studies examining patient perspectives on clozapine treatment,23–26 have been included.18 19 21 However, since the publication of the protocol article for this study,27 two systematic reviews on patient perspectives have been published,28 29 reporting on a further 16 studies.30–45
Nevertheless, our preliminary literature search revealed that additional studies on both patients’ and clinicians’ perspectives on clozapine treatment exist, without having been included in any of the existing systematic reviews.
Consequently, it is difficult to conclude if the lack of change in clozapine prescribing is due to an inadequate dissemination and/or implementation of current evidence or due to an inadequate elucidation of key aspects of the topic. A broader and more comprehensive overview of the literature addressing patients’ and clinicians’ perspectives on clozapine treatment is therefore warranted.
Key definitions
Patients
The term ‘patients’ refers to adult (age ≥18 years) patients affiliated with somatic or mental health services.
Clinicians
The term ‘clinicians’ will be used for all clinical staff affiliated with somatic or mental health services treating adult patients.
Objectives
In line with the original framework by Arksey and O’Malley,46 we aimed to conduct a scoping review in order to (a) investigate the extent and variety of primary studies covering patients’ and/or clinicians’ perspectives of clozapine treatment and (b) to identify gaps in the current research. A secondary aim was to (c) summarise the key findings related to patients’ and clinicians’ perspectives on clozapine treatment.
Methods and analysis
The protocol for this scoping review27 was designed in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA)47 to ensure that all relevant information for the future scoping review could be included. It was guided by the corresponding Joanna Briggs Institute guidelines ‘guidance for conducting systematic scoping reviews’48 and the ‘updated methodological guidance for the conduct of scoping reviews’.49 We have furthermore sought complemental guidance in the advanced framework recommendations by Levac et al.50
A completed PRISMA checklist for the reporting of scoping reviews has been submitted with the manuscript.
Registration
PROSPERO does not offer registration of scoping reviews; however, the review protocol was prospectively registered with the Open Science Framework (OSF), registration DOI 10.17605/OSF.IO/5K4S351 and subsequently published as an open-access article.27
Eligibility criteria
Publications were considered eligible for inclusion if they met the following selection criteria:
Inclusion criteria
Published in the English language.
Primary, empirical literature addressing patients’ or clinicians’ perspectives on clozapine treatment (including both peer-reviewed research papers and grey literature such as conference abstracts and dissertation papers).
Exclusion criteria
Non-empirical literature (ie, editorials, opinion and discussion papers) and case reports.
Secondary studies (reviews/overviews); however, their reference lists were included for citation tracking.
No limitation has been set for year of publication or type of primary study.
Rationale
We chose to limit our search to empirical, primary studies. The rationale for this was our aim to map the scope of studies on perspectives/attitudes/perceptions, with the intent to identify gaps in the existing scientific evidence. However, in order to uphold a certain level of scientific evidence, we disregarded case reports as sources of evidence.
We considered non-empirical data and secondary studies irrelevant to the objective of this review.
Due to feasibility resources, the language was restricted to English.
Information sources
The electronic databases Cochrane Library, CINAHL, Web of Science, PsycINFO, MEDLINE and EMBASE were searched for relevant publications, supplemented with a booster search on Google Scholar. This combination of sources has previously been reported to guarantee adequate and efficient coverage.52
Furthermore, The Networked Digital Library of Theses and Dissertations (NDLTD) and OpenGrey were searched for additional relevant literature, and citation tracking of selected studies was undertaken.
Search
The search strategy used for this scoping review was developed by the lead investigator (author MIJ) in collaboration with an experienced research librarian. In accordance with established scoping review methodology,48 49 it consisted of three steps: First, an initial search of selected databases, in this case MEDLINE and EMBASE, was performed. Search terms included but were not restricted to, clinician, doctor, psychiatrist, patient, consumer, perspective, experience, attitude, perception, clozapine, (leponex) and (clozaril). The search was then followed by screening of the identified articles for relevant text words and index terms.
Second, the search was refined, incorporating the identified keywords and index terms. Search terms were adapted to the requirements of each selected database.
Table 1 shows the refined electronic search strategy for Embase (originally published with the scoping review protocol).27 After completing the refinement across all selected databases, a second search (the actual search), was undertaken by 25 June 2021. A Google Scholar booster search (screening only the first 200 hits and related titles) was undertaken by 23 May 2022.
Electronic search strategy for Embase
The third step of the search strategy consisted of searching the additional sources of literature (NDLTD and OpenGrey) on 9 June 2022, as well as handsearches of the reference lists of all included studies and excluded review/overview studies, to ensure a comprehensive literature identification.
A final Google Scholar search was conducted after the completion of the study (on 3 January 2024) to ensure the relevancy of the study results. Studies identified in the postanalysis Google Scholar search were not included in the study results but are reported and commented on as part of the discussion.
Selection of sources of evidence
Records from the database search and from additional sources were imported to the reference management software EndNote53 and duplicates were removed. The merged search results were then exported to Covidence.54 Two individual reviewers (authors MIJ and JPS) screened the titles and abstracts of all identified studies, followed by full-text screening according to the inclusion and exclusion criteria. Full texts were searched for, for all studies included for full-text screening. The search for full texts included assistance from a research librarian and requests for full texts on ResearchGate. If no full texts were available, studies were included for full-text screening in the form available. Any disagreement related to study eligibility at either stage was resolved through discussion between the two reviewers. Doubts regarding overall eligibility were solved by the involvement of a third party; for example, we realised, during the screening phase, that some previous reviews/overviews had included studies reporting quantitative measures of well-being or quality of life. Through discussion with a third party (author OJS), we deemed such measures too influenced by factors other than the present use of APs and, therefore, not an assessment of perspectives on clozapine treatment. This decision led to the exclusion of studies reporting only these measures.
Data charting process
Microsoft Excel,55 Covidence and EndNote were used to manage the screening process and to organise the data.
A refined data extraction form, based on the preliminary form developed for the study protocol, was constructed in Covidence.
The preliminary data extraction form was refined to include predefined content categories (perspectives on active clozapine treatment/discontinued clozapine treatment/initiation of treatment) and subcategories (eg, satisfaction with/perceived burden of/perceived efficacy of treatment) describing the perspectives being explored. The predefined categories and subcategories were constructed based on the observations made during the screening process. The subcategory ‘other’ was added for additional content not covered by the predefined subcategories.
In accordance with the directed approach to content analysis,56 the coding of study content was performed during the data extraction phase.
The content of ‘other’ was coded later on, during the analysis phase, to form additional subcategories.
The extraction form was piloted twice, on 10 studies, by both reviewers. After each pilot round, it was refined to record the information needed to answer the research questions. The data were extracted in doublet by the two reviewers and then combined into a consensus extraction. The lead investigator resolved any disagreements.
Data items
In the data charting phase of the review, the following information was collected:
Author(s).
Year of publication.
Title.
Country of origin.
Type of study.
Study population and study context/relevant characteristics (mixed staff/psychiatrists only, clozapine/non-clozapine/mixed patients, inpatients/outpatients, etc).
Relevant outcome measures and method of assessment (any assessments of attitude or perception of clozapine treatment, eg, measures of treatment satisfaction or efficacy, perception of barriers to its usage, reasons for treatment withholding or refusal, perceptions of initiatives for increased treatment utility).
The content category(s) and subcategories of explored perspectives (perspectives related to active/discontinued/not yet commenced clozapine treatment? Perspectives related to specific patient cases or in general? Satisfaction with treatment?/burden of treatment?/efficacy of treatment?, etc).
Key findings related to patients’ or clinicians’ perspectives on clozapine treatment.
Synthesis of results
A narrative summary of the results has been accompanied by visual aids.
Studies were grouped and mapped based on the content categories of explored perspectives (eg, perspectives relating to active clozapine treatment/discontinued clozapine treatment/clozapine commencement) and to the origin of perspectives (eg, patients’/clinicians’ perspectives) to provide a graphical presentation of clusters and gaps in the existing evidence. A table presents an overview of basic study characteristics, while an online supplementary spreadsheet presents an in-depth overview of individual study data (eg, author, year, methods and key findings). Furthermore, simple column charts present distributions of explored subcategories of perspectives.
In line with the review’s objectives, recommendations for future investigations based on the identified pattern of previous research have been included in the conclusion.
Differences between the methods described in the published protocol and the methods used in the final review
In the protocol, we defined ‘patients’ as adult patients affiliated with mental health services due to psychiatric disorders, while ‘clinicians’ were defined as ‘psychiatrists’, that is, medical doctors affiliated with mental health services. However, during the screening process, we observed that several studies reported on perspectives from mixed groups of patients, including patients with organic psychoses. These patients were sometimes, but not always, affiliated with mental health services which would entail an inconsistent inclusion or exclusion of several sources of evidence. In the same way, we observed that several studies of clinician perspectives included mixed groups of clinicians, not all of them distinguishable in terms of psychiatrist versus non-psychiatrist perspectives — and not all of them including psychiatrists. Furthermore, psychiatrists are not the only prescribers of clozapine. Therefore, we changed the definitions to the current ones (‘patients’ = adult patients affiliated with somatic or mental health services; ‘clinicians’ = clinical staff treating adult patients) to ensure that all relevant studies were included for mapping.
Results
Selection of sources of evidence
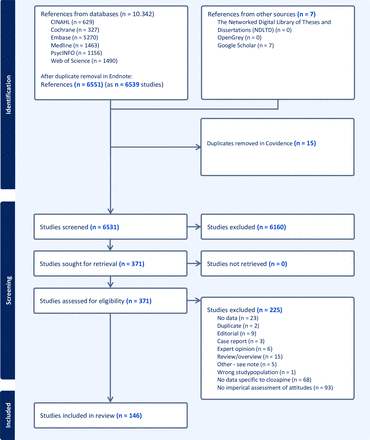
The initial search identified 6531 studies after duplicate removal. At the title and abstract screening stage, another 6160 studies were excluded. A total of 371 studies were screened as full texts (or at full-text level), and a further 225 studies were excluded for reasons outlined in the PRISMA flow chart (figure 1).
Screening. PRISMA flow chart outlining the process of screening and inclusion of eligible sources of evidence. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
General characteristics of included sources of evidence
In total, 146 studies,23–26 30–40 42–45 57–183 represented by 158 sources of evidence23–26 30–40 42–45 57–195 and 57 countries across 6 continents, were included. The majority of the studies (n=132, 90%) were from Western countries which, for the purposes of this study, are understood as the USA, Europe, Canada, Australia, Latin America and New Zealand.
One-hundred-fifteen studies were extracted as articles, 2 as reports, 14 as short communications, 1 as an article summary, 13 as abstracts and 1 as a conference poster. The year of publication was between 1990 and 2021. One hundred thirty-eight studies used a quantitative design (ie, survey questionnaires, rating scales, case-note reviews, etc), 13 used a qualitative design (interviews), 1 study used both and 6 studies used a mixed-methods design. Online supplemental table 1 displays a list of the included studies and selected study characteristics.
Supplemental material
Individual, in-depth study characteristics and key findings are provided in the ‘Individual study data’ (dataset),196 retrievable from the Open Science Framework repository at https://doi-org.ezproxy.u-pec.fr/10.17605/OSF.IO/8T5PV.
Thirty-nine of the studies23–26 30–40 42 44 45 72 86 99 100 102 105 109 110 117 119 122 130–132 134 136 137 161 168 182 188 (27%) have previously been included in systematic reviews18–22 28 29 addressing patients’ and/or clinicians’ perspectives on clozapine treatment.
Distribution of studies based on categories of explored perspectives
Figure 2 provides a graphical presentation of the distribution of studies.
Graphical presentation of the scope of studies reporting on patients’ and/or clinicians’ perspectives on clozapine treatment. The sum of studies depicted in row 2–4 does not correspond the total number (NTotal) of included studies (row 1). Some studies report perspectives on more than one content category (eg, on both active treatment and initiation), that is, the same study may be counted multiple times, representing different content categories (rows). Some studies report on perspectives from more than one study population (eg, both patient and clinician perspectives). The number of studies reporting ‘overlapping’ perspectives from different populations is shown in the intersection between two ovals. In several studies, the reported perspectives could not be assigned to either patients or clinicians, for example, due to the secondhand origin of data from case files. The origin of perspectives in these studies is labelled as ‘unsure’. *The number of studies reporting case-specific psychiatrist perspectives.
Of the 146 included studies, 39 reported patient perspectives,23 25 30–39 42–44 57–80 9 reported both patient and clinician perspectives24 26 40 45 81–85 and 54 reported clinician perspectives.86–139 In 45 studies,139–183 the patient/clinician origin of some or all of the reported perspectives was unclear (figure 2, row 1).
Of the total 63 studies reporting clinician perspectives, 34 reported perspectives belonging to psychiatrists,86–93 95–97 102 103 106 107 109 113 116 119 122–132 134–137 4 reported perspectives belonging to non-psychiatrists only40 94 98 138 and 22 reported perspectives belonging to a mixture of different clinicians.26 81 83–85 88 99–101 104 105 108 110–112 114 115 117 118 121 133 139 In four studies,24 45 82 120 the type of clinicians was unclear (figure 2, row 1). Fifty-seven studies (90%) were from Western countries (online supplemental table 1).
Of the total 48 studies reporting patient perspectives, 16 reported on perspectives belonging to schizophrenia patients in specific,30 31 36 38 40 44 59 64 66 71 72 76–79 84 2 on non-schizophrenia patients only,68 75 18 on patients with a variety of diagnoses24–26 35 37 39 42 43 58 61–63 69 70 73 74 80 83 and in 12 studies23 32–34 57 60 65 67 81 82 85 193 the type of patients was unclear (figure 2, row 1). Forty-one studies (85%) were from Western countries online supplemental table 1.
Seventy-three studies reported perspectives related to active/ongoing clozapine treatment23–26 30–40 43–45 57–77 79 81–85 89–96 98 100 102–104 108 110 115 117 119 122 126 130 132 133 138 151 159 178 181 (figure 2, row 2).
Forty-five of the studies (62%) reported on clozapine patients’ attitudes towards their treatment,23–26 30–40 43–45 57–77 79 81–85 of which 15 studies reported specifically on experiences belonging to schizophrenia patients.30 31 36 38 40 44 59 64 66 71 72 76 77 79 84
Thirty-three studies (45%) reported clinician perspectives on clozapine treatment,24 26 40 45 81–85 89–96 98 100 102–104 108 110 115 117 119 122 126 130 132 133 138 of which three reported clinician perspectives on clozapine treatment in relation to specific patient cases.40 90 103
In four studies (5%),151 159 178 181 the patient/clinician origin of perspectives on active treatment was unclear.
Fifty-three studies reported perspectives on clozapine discontinuation34 79 80 90 116 118 120 125 139–183 (figure 2, row 3).
Three of these studies (6%) reported on patients’ reasons for discontinuing clozapine treatment,34 79 80 and five studies (9%) reported clinician-perceived reasons for clozapine discontinuation,90 116 118 120 125 all of them in specific patient cases.
Forty-five studies (85%) reported reasons for discontinuation but could not be assigned to either patient or clinician perspectives due to the case-note origin of the data.139–183
Fifty-three studies reported perspectives on clozapine initiation42 78 83 86–93 95–102 104–107 109–114 117 118 120–124 126–132 134–137 139 148 155 160 163 190 (figure 2, row 4).
Three studies (6%) reported on patients’ attitudes towards clozapine commencement,42 78 190 whereas 46 studies (87%) reported on clinicians’ perspectives on clozapine initiation.83 86–93 95–102 104–107 109–114 117 118 120–124 126–132 134–137 139 In five of these studies,90 118 120 134 139 the clinician perspectives were related to specific cases of clozapine initiation.
In four studies (8%),148 155 160 163 the patient/clinician origin of perspectives was unclear.
Characteristics of explored patient perspectives
In the 45 studies on clozapine patients’ attitudes towards their medication,23–26 30–40 43–45 57–77 79 81–85 10 different subcategories of perspectives were explored (online supplemental figure 1a). The most frequently explored subcategories were the ‘perceived burden of treatment’ (in 36 studies) and ‘perceived efficacy of treatment’ (in 34 studies).
Supplemental material
The patients’ perspectives on clozapine treatment were overall positive, with the majority of patients expressing high efficacy—and satisfaction ratings with clozapine and a preference for clozapine compared with their previous treatment—regardless of diagnosis. Downsides such as adverse side effects and the need for blood monitoring were generally accepted among all groups of patients due to the perceived efficacy of clozapine treatment. However, patients tended to prefer point-of-care (POC) devices for finger prick blood sampling over conventional venipuncture—as well as other interventions aiming at simplifying treatment (the categories of assessed interventions are shown in online supplemental figure 1b). Patients with organic psychosis/neurological conditions seemed more vulnerable to adverse side effects and to the logistic barriers to monitoring (‘Individual study data’,196 key findings, at https://doi-org.ezproxy.u-pec.fr/10.17605/OSF.IO/8T5PV).
In the three studies reporting on patients’ perspectives on clozapine discontinuation,34 79 80 the reasons for discontinuation were explored. Five categories of reasons were mentioned (online supplemental figure 2), with ‘adverse side effects’ and ‘lack of efficacy’ being the most frequently mentioned ones (in three and two studies, respectively). Lack of efficacy was the most prominent reason in two out of the three studies79 80 (‘Individual study data’, key findings196).
Supplemental material
In the three studies on patients’ perspectives on clozapine commencement,42 78 80 six subcategories of perspectives were explored (online supplemental figure 3). The subcategory ‘patients’ reasons for refusal’ was, as the only subcategory, assessed in two studies42 78; however, only fully reported on in one of them—a study on acutely unwell inpatients with schizophrenia or schizoaffective disorder.42 The prospect of admission as a requirement for clozapine commencement seemed to be the main barrier to acceptance for most patients in this study. Blood monitoring was not the most prominent reason for refusal in any of the two studies (‘Individual study data’, key findings196).
Supplemental material
Characteristics of explored clinician perspectives
In the 33 studies on clinician perspectives on active clozapine treatment,24 26 40 45 81–85 89–96 98 100 102–104 108 110 115 117 119 122 126 130 132 133 138 13 different subcategories of perspectives were explored (online supplemental figure 4a). The most frequently explored subcategories of perspectives were ‘perceived efficacy of treatment’ (in 18 studies) and ‘utility of/preference for a clozapine facilitating intervention’ (in 16 studies). In the latter, eight different categories of interventions aiming at improving treatment utility, satisfaction or adherence were assessed (online supplemental figure 4b).
Supplemental material
Most studies on clinician perspectives in relation to active clozapine treatment were studies of general perspectives (30 studies); however, three studies40 90 103 reported clinician perspectives in relation to specific patient cases of clozapine treatment (figure 2, row 2). The subcategories of explored perspectives in these studies were ‘efficacy of treatment’ (in all three studies) and ‘perceived burden of treatment’ (in one study).
Overall, the clinicians perceived clozapine to be efficient, but of great burden to the patients in terms of adverse side effects and blood monitoring requirements. The clinicians were consistently in favour of POC devices for capillary haematological monitoring over conventional venous sampling as this was considered to increase both patient and clinician satisfaction with treatment. Other types of facilitating interventions were also considered relevant (although evaluated less frequently) or, as in the case of clozapine clinics, with mixed enthusiasm (‘Individual study data’, key findings196).
In the five studies on clinician perspectives on clozapine discontinuation,90 116 118 120 125 the reasons for clozapine discontinuation were assessed. In total, nine different categories of reasons were mentioned (online supplemental figure 5), of which ‘no/poor response’, ‘patient’s refusal to take clozapine and/or do bloodwork’, ‘non-compliance with clozapine’ and ‘adverse side effects’ were the most frequently mentioned ones, each in four studies. However, ‘adverse side effects’ was the most prominent reason in most studies (n=3), and ‘non-compliance with clozapine’ in two studies. See ‘Individual study data’, key findings,196 for more information on individual study findings.
Supplemental material
Forty-six studies explored clinician perspectives on clozapine initiation,83 86–93 95–102 104–107 109–114 117 118 120–124 126–132 134–137 139 most of them (n=41) in general terms (figure 2, row 4).
Thirteen subcategories of perspectives were explored (online supplemental figure 6a), the most frequent ones being ‘barriers to treatment initiation’ (in 29 studies) and ‘utility of /preference for a clozapine facilitating intervention’ (in 23 studies). Eighteen different categories of barriers to initiation were mentioned (online supplemental figure 6b), of which ‘concerns about adverse side effects’ were the most frequently mentioned one (in 20 studies). Seventeen subcategories of interventions were mentioned as facilitators of clozapine initiation (online supplemental figure 6c), most studies referring to facilitators such as ‘more training in clozapine treatment’ (n=6) and ‘outsourcing of clozapine initiation (n=6), monitoring and/or prescribing’ (n=6).
Supplemental material
Five studies explored clinicians’ perspectives on clozapine initiation in specific patient cases,90 118 120 134 139 two of them reported perspectives belonging to psychiatrists in specific90 134 (figure 2, row 4). The subcategories of explored perspectives in the case-specific studies were ‘reasons for treatment withholding’ (in four studies), ‘utility of /preference for a clozapine-facilitating intervention’ (in two studies), ‘barriers to initiation’ (two studies), ‘other: reason for inpatient initiation’ (in one study), ‘other: indication for treatment’ (one study) and ‘other: clinical practice’ (one study).
Two of the case-specific studies relied on case note data,120 139 while three studies used a more direct approach to assessment.90 118 134 In two of these ‘direct’ studies,118 134 the patients were inpatients and in the third study90 the patients’ status as inpatients or outpatients was missing.
In the four case-specific studies exploring ‘reasons for treatment withholding’,118 120 134 139 six categories of reasons were mentioned (online supplemental figure 6d). The most frequently mentioned ones were ‘expected non-compliance with either drug or blood monitoring’, ‘risks outweigh benefits’ and ‘somatic issues’ (each of them mentioned in two studies).
Data on psychiatrists’ reasons for clozapine withholding in specific cases were only reported in one study, a study of inpatients,134 in which the expected patient refusal or non-compliance with either drug or blood monitoring were the most frequently stated reasons.
Discussion
Summary of evidence
With this scoping review, we aimed to map the scope of primary studies reporting on patients’ and/or clinicians’ perspectives on clozapine treatment. The map was intended to provide an overview of the number and distribution of studies exploring different categories of perspectives, and, hence, of clusters and gaps in the existing research.
We included 146 studies, of which only 39 (27%) had been included in previous systematic reviews addressing patients’ and/or clinicians’ perspectives on clozapine treatment. We found that the research within this area for most parts was on Western populations and clustered around the same topics: Most studies on the subject could be assigned to clinician perspectives (n=63, 43%) and most of these studies (n=41, 65%) centred around general (ie, non-case-specific) perspectives on clozapine initiation. Forty-eight studies (33%) reported on patient perspectives, the vast majority of them (n=45, 94%) on clozapine patients’ attitudes towards their ongoing treatment.
In contrast, only a few studies reported on patients’ perspectives on clozapine commencement (n=3, 2%) or discontinuation (n=3, 2%), or on clinicians’ perceived reasons for non-clozapine treatment in specific patient cases (n=5, 3%).
Efficacy and burden of treatment were the most frequently assessed subcategories of perspectives in studies on active clozapine treatment. Clozapine patients across countries and diagnoses were overall satisfied with clozapine treatment due to its efficacy. Side effects and monitoring requirements were considered of minor importance. Clinicians across professions found clozapine treatment efficacious but a burden to the patients due to the adverse side effects and monitoring requirements. The utility of treatment-facilitating interventions was also frequently assessed. In studies on clinicians’ general perspectives on clozapine treatment, this was the second most assessed subcategory. Both clozapine patients and clinicians considered finger-prick blood sampling more convenient than conventional blood sampling.
Reasons for clozapine discontinuation were assessed in both patient and clinician studies. The primary reason for discontinuation was listed as poor response in patient studies whereas clinicians most frequently attributed the discontinuation to adverse side effects.
In studies assessing perspectives on clozapine initiation, most patient studies explored individual reasons for clozapine refusal whereas clinician studies mainly explored general perspectives on barriers to initiation. To patients, the most prominent reason for refusing clozapine commencement was the prospect of hospital admission; in clinician studies, concerns about adverse side effects and/or concerns about patient adherence/refusal to treatment and monitoring were reported the most.
The discrepancies and similarities between patient and clinician perspectives on active clozapine treatment have also been described in previous reviews restricted to studies on schizophrenia and related disorders18 19 21 but seem applicable to a range of patient groups. The generalisability of the apparent discrepancies between patient and clinician perspectives on discontinuation and initiation summarised above is, however, doubtful due to limitations of the evidence. In line with established scoping review conventions,46–50 no formal critical appraisal of the included studies has been conducted. Nevertheless, several issues regarding the scope and characteristics of studies must be addressed: of the five case-specific studies on clinician-perceived reasons for non-clozapine treatment, only three studies used a direct approach to the assessment of reasons; the others relied on case-note data. Moreover, only one of these studies reported specifically on psychiatrists’ reasons for treatment withholding, and no studies could elucidate the reasons for clozapine withholding for clozapine-eligible outpatients—only for inpatients. The reasons for treatment withholding for inpatients might not reflect the reasons for treatment withholding for outpatients, and the reasons mentioned most frequently in studies of clinicians’ general perspectives on barriers to clozapine treatment may not reflect the most decisive reasons in real-world practice. Therefore, the prescribing clinicians’ reasons for clozapine withholding in individual real-world cases must be explored to clarify this matter.
Of the three studies on patient attitudes towards clozapine commencement, only one study assessed the patients’ willingness to try clozapine and the factors affecting their choice. As with the studies on clinicians’ reasons for withholding, this was a quantitative study on inpatients. The attitudes of inpatients may differ from the attitudes of outpatients, and quantitative (closed) questions on previously unexplored matters may not provide the depth of information needed. Thus, in order to understand the barriers and facilitators of clozapine initiation, the perspectives of prospective recipients must be thoroughly explored across different settings. This requires both in-depth qualitative assessments and the use of similar/uniform questions to enable comparison among studies.
Lastly, a substantial number of studies reported on reasons for clozapine discontinuation (n=53, 36%); however, due to the second-hand or even third-hand origin of data from case notes, the reported reasons could not be assigned to either patients or clinicians in most studies (n=45, 85%). In some studies, the main reasons were divided into patient and clinician categories; however, this division was a matter of interpretation by the authors themselves. Consequently, only a few studies reported the results of a direct assessment of patients’ or clinicians’ perspectives on clozapine discontinuation—and none of them qualitatively.
Clinical implications and recommendations for future studies
There are several indications for clozapine treatment, the main indication being treatment-resistant schizophrenia.7 Approximately one-third of all patients with schizophrenia are treatment-resistant5; however, far less than one-third of patients with schizophrenia are treated with clozapine.13 15 Furthermore, clozapine is often trialled late in the treatment course, after several more AP trials than the two or three trials recommended in guidelines.12 The clozapine avoidance or postponement leaves the patients insufficiently treated for an elongated period of time (provided that they would respond to clozapine treatment), and at risk of poorer response to clozapine when finally commenced. Previous research has shown that the chances of responding to clozapine treatment decrease with time and with the number of ineffective AP trials prior to clozapine commencement.197 As this review underpins, clozapine underutilisation has been a concern for decades and a vast number of studies have sought to assess patients’ and/or clinicians’ experiences or perceptions of clozapine treatment, the clinicians’ perceived reasons for barriers or facilitators of its usage as well as the reasons leading to clozapine discontinuation. Despite that, clozapine continues to be used too little and too late.19 One explanation for the lack of change in clozapine utility could be the repetitive pattern of previous research described in this review. We reckon that the evidence of clinicians’ general perspectives on barriers to clozapine prescribing has met its saturation point and that both patient and clinician perspectives on active clozapine treatment are well documented by now, at least for Western populations. Additional studies in these areas will merely repeat what is already known—although still highly relevant to assess in a clinical context.
In contrast, there is an apparent worldwide gap in the evidence on clozapine-naïve patients’ attitudes towards clozapine commencement, particularly the attitudes of eligible outpatients as the evidence in this regard is completely absent. The same applies to direct (ie, not case-note derived) assessments of clinicians’ reasons for clozapine withholding in individual cases of eligible outpatients. These kinds of ‘real-world’ studies might provide the information needed for a successful increase in clozapine utilisation.
Furthermore, due to the limited number of studies directly assessing patients’ and clinicians’ perspectives on clozapine discontinuation, future studies exploring both the patients’ and the clinicians’ needs in terms of clozapine discontinuation vs continuation or rechallenge in individual patient cases are warranted as they could provide valuable insights for treatment optimisation.
Since the beginning of this work, we have conducted our own studies to address some of the identified gaps in research, including a mixed-methods study on case-specific clinician reasons for clozapine withholding198 and a mixed-methods study on attitudes of clozapine-naïve outpatients toward clozapine commencement (in review). Furthermore, a renewed Google Scholar search conducted in January 2024 revealed the publication of two new (qualitative) studies on patient perspectives towards clozapine discontinuation.199 200 No other published studies within the identified underprioritised areas of research were found. Instead, eight additional studies on general clinician perspectives on clozapine treatment,101 201–207 three additional studies on clozapine-patients’ (and their clinicians’) attitudes towards their ongoing treatment,41 208 209 and three additional studies on case-note-derived reasons for clozapine cessation or withholding210–212 appeared, ratifying the repetitive behaviour of current research and the importance of disseminating these review results in order to direct future research toward more meaningful and less well-described areas.
Strength and limitations
Strengths: This scoping review has been developed following an established scoping review methodology to ensure methodological rigour. The prospective registration and publication of its peer-reviewed protocol have ensured transparency of the review process. The three-stepped search strategy, involving a broad range of search terms and relevant databases and sources of grey literature, has ensured a comprehensive search of the literature, and the multiple booster searches on Google Scholar have ensured a continued update on the scope of literature.
Limitations: The comprehensive search strategy, including terms such as perspective, attitude and subjective, entailed the risk of identifying an overwhelmingly large amount of literature. As a means to ensure a certain level of scientific evidence in the included studies, we deemed the exclusion of data sources such as case reports, commentaries and letters expressing individual opinions necessary.
To delimit the study and ensure comprehensiveness in the reporting, we confined our search to studies on adult patients and clinicians treating adult patients. However, the perspectives of caregivers and perspectives related to clozapine treatment of children and adolescents are also important and the search for such studies would have made an interesting extension to the map.
Furthermore, due to feasibility reasons, the search was restricted to publications in English though studies providing résumés, abstracts or posters in the English language were included, even if the full texts were retrievable only in the native language.
All these pragmatic decisions may have precluded the identification of some relevant insights and studies.
Conclusions
A substantial number of studies reporting on patients’ and/or clinicians’ perspectives on clozapine treatment were identified, most studies without previous inclusion in systematic reviews. The mapping of studies revealed a repetitive behaviour of current research with most studies clustered around topics such as clinicians’ general perspectives on active clozapine treatment, clinicians’ general perspectives on barriers to clozapine initiation, and, to a lesser extend, clozapine patients’ attitudes towards their ongoing treatment. This behaviour leaves three distinct gaps in research: (1) studies exploring clozapine-eligible, yet clozapine-naïve, patients’ attitudes towards clozapine commencement, (2) studies exploring clinicians’ reasons for clozapine withholding and perceived facilitators of clozapine treatment in individual, real-world patient cases, and (3) direct (ie, not case-note-derived) assessments of both patient and clinician perspectives on clozapine discontinuation, continuation and rechallenge in individual patient cases.
Future studies within these underprioritised areas of research might provide the evidence needed to turn clozapine from underutilisation.
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. The data supporting the findings of this study are retrievable from the data repository The Open Science Framework (OSF) at https://doi-org.ezproxy.u-pec.fr/10.17605/OSF.IO/8T5PV in the file 'Individual study data'196 or available as supplementary materials attached to the article.
Ethics statements
Patient consent for publication
Ethics approval
Not required.
Acknowledgments
We thank our research librarian, Trine Kæstel, Psychiatric Research Unit, Region Zealand Psychiatry, Denmark, for assisting with the search strategy.
References
Footnotes
Contributors MIJ, OJS, SFA, JN and ES participated in conceptualising the review. MIJ developed the search strategy in consultancy with a research librarian (see 'Acknowledgements'), wrote the protocol, performed the search and managed the data. JPS and MIJ performed the screening and data extraction in collaboration; OJS acted as a third-party consultant in cases of doubt. MIJ wrote the manuscript. All authors have critically reviewed the manuscript and approved the final version for submission. MIJ is the guarantor of this work.
Funding This work is part of a Ph.D. project funded by the Mental Health Services of Region Zealand Psychiatry East, Roskilde, Denmark, The Psychiatric Research Unit, Region Zealand Psychiatry West, Slagelse, Denmark, and the Psychiatric Centre Glostrup, Unit for Complicated Schizophrenia, Mental Health Services of The Capital Region of Denmark, in collaboration. Numbers Grant are not applicable. Region Zealand Psychiatry received, from a former patient, a bequeathed donation favoring patient-oriented research within the region. The Ph.D. project in question, and hence this study, is partially funded by that donation.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.