Article Text
Abstract
Objectives To investigate uterine fibroid (UF)-associated imaging changes, and their prevalence, incidence and potential risk factors in the Chinese population.
Design This was a retrospective observational study using health examination data.
Setting A physical examination centre in Nanchong, China, between October 2017 and December 2020.
Participants A total of 33 915 Chinese women older than 15 years of age underwent uterine imaging during the study period.
Primary and secondary outcome measures This study identified entries of UF-associated imaging changes through a two-round expert consultation and calculated prevalence and incidence of UF-associated imaging changes. Logistic regression estimated the association (OR, 95% CI of body mass index, high blood pressure (HBP), blood lipid profile, and fasting blood glucose level) with UF-associated imaging changes. Age-stratified (≤40 years and >40 years) risks were ascertained.
Results Besides the entry ‘Potential UF’, 17 other entries of UF-associated imaging changes screened by the expert consultation were included, involving a total of 46 864 records (n=33 915), and crude prevalence=25.18%; crude incidence density/1000-woman-years=63.28. Incidence and prevalence increased with age during reproductive age (15–49 years) and decreased thereafter. The greatest burden was in women aged 40–54 years, the prevalence was 38.60%–45.38% and the incidence was 14.73%–17.96%. In the incident younger population (age ≤40 years), overweight (OR: 1.48, 95% CI 1.03 to 2.14) and HBP (OR: 2.16, 95% CI 1.10 to 4.24) were associated with a higher risk for UF-associated imaging changes; in the >40 years group, no association was observed.
Conclusion UF incidence and prevalence in Asians were higher than previously reported, showed age-related increase in reproductive age, and UF incidence increased with overweight and HBP in ≤40-year-old participants. Variation in UF burden and factors with higher risk noted in different age ranges, and the correlations identified in younger women make it possible for early preventive measures for women with a higher risk of UF.
- China
- Gynaecological oncology
- PREVENTIVE MEDICINE
- EPIDEMIOLOGY
- Retrospective Studies
Data availability statement
Data are available upon reasonable request. The anonymised participant data sets and data collection forms generated during and/or analysed (online supplemental files 1; 2) during this study are available from the corresponding author. The funder, investigators and collaborators will review and approve the proposals based on scientific merit and submit them to the Ethics Committee of Nanchong Central Hospital, the Second Clinical Medical College, North Sichuan Medical University (2021-004). After the proposal has been approved for a long period of time, data can be shared along with anonymised participant data accompanied by a data dictionary through a secure online platform after signing a data access and confidentiality agreement.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This was a retrospective study based on regular health examinations population that included a large number of asymptomatic women.
The study is based on a larger population and reveals the data on the incidence and prevalence of uterine fibroids (UF) in the Asian population to some extent.
Incidence analysis used the high blood pressure (HBP) status before the incidence of UF, suggesting HBP was associated with a higher risk for UF-associated imaging changes.
The study was conducted in a mostly urban population that actively visited hospitals for health check-ups. Therefore, the conclusions cannot be extrapolated to the epidemiological characteristics of UF in rural populations.
This analysis was based on real-world practice, which did not include certain commonly recognised factors that are related to UF, such as behaviour lifestyle, menstrual and reproductive history, or family history.
Background
Uterine fibroids (UFs) are the most common benign tumours in women of reproductive age1 and may cause abnormal uterine bleeding, pelvic pain and malpresentation that necessitates caesarean section2 3 and even lead to hysterectomies, with a rate of surgical or procedural treatment for UF of 30,4 which shows that UFs can affect the quality of life. UF incurs substantial direct and indirect costs.5 UF is estimated to confer costs of $5.9–$34.4 billion annually in the USA,6 Germany spends nearly €212 million a year, France spends €73 million, and the UK spends €53 million.7 In China, UF-associated disability-adjusted life years reached 281 976.67, and the UF burden increased rapidly during 1996–2016.8 Globally, even though the age-standardised disability-adjusted life year (DALY) rate of UF decreased, the age-standardised incidence rate increased from 1990 to 2019,9 and underdeveloped countries were disproportionately affected by the increased health burden.10 Understanding the epidemiological characteristics of UF is crucial for allowing early detection and treatment of UF, thereby preserving fertility, and improving the quality of life of women as well as reducing the disease burden.
Existing studies have shown that the reported prevalence and incidence of UF varied across populations. UF prevalence and incidence are higher in African-American women than in other races. Studies reported a prevalence of 35.7% in the black or African-American population, 10.7% in the white population,11 and the rate was consistently higher among women of African race.12 Incidence rates in the USA and Europe range from 845 to 3745 cases per 100 000 women-years and the prevalence ranges from 4.5% to 68.6%.13 In South Korea, women’s UF prevalence ranged from 2.43% to 42%.14 15 Race is also the most frequently reported risk factor for UF. However, in Asia, a limited number of studies were specifically designed to examine the epidemiological characteristics of UF,16 17 which is true especially for China. A recent cross-sectional study noted that the prevalence of UF in Chinese women aged 30–55 years was 8.5% (7.8%, 9.2%), but failed to explore incidence and risk factors.18
In most previous studies, an imaging-based UF diagnosis depends on ultrasound and MRI, and ultrasound is adequate to enable their diagnosis (90%–99% sensitivity).19 In China, ultrasound is a commonly used method for diagnosing UF.20 As most patients don't undergo pelvic imaging examination until the symptoms have started impairing their daily life, symptoms have been reported only in 30% of patients with UF in early stage disease. Thus, data from regular gynaecological clinics usually result in an overestimation of the UF prevalence,21 while data from the general population may result in underestimation as asymptomatic patients would not be identified without any medical examination. According to the results of a previous study, the incidence of UF was 33% by clinical assessment, 50% by ultrasound and 77% by histological assessment.22 The annual health check-up in China routinely includes pelvic imaging examinations for women of reproductive age.23 Among the 348 health examination centres within 30 provinces in China in 2018, the average annual number of health examinations per centre was approximately 50 000.24 Thus, health examination data cover a diverse population and comprise records of regular examination results for many years. Pelvic ultrasound has been commonly used in the regular examinations for women, even if they do not report any symptom related to UF. Therefore, health examination data make it possible to estimate the prevalence and incidence of UF, enabling epidemiological characterisation25 and could help identify risk factors in the real world, which would support strategies for disease control and management.26 27 However, the analysis of real-world health examination data involves many challenges.28 29
Using multiyear health examination data of pelvic imaging of women in western China, we aimed to confirm the UF-associated imaging changes, characterise the prevalence and incidence, analyse the age distribution of the incidence and prevalence, and explore the potential risk factors for UF-associated imaging changes in the Chinese population. The ultimate goal is to explore the disease burden and to support preventive strategies for those who are at risk of UF.
Methods
Study data
We conducted a population-based retrospective cohort study using real-world data, and extracted data of women who attended regular health examinations from October 2017 to December 2020 at Nanchong Central Hospital, which is located in western China. In China, regular health check-up refers to the medical practice of physical examination of the examinee through medical means and methods, with the purpose of understanding the examinee’s health status, and early detection of clues of diseases and hidden health problems.30 The inclusion criteria were the presence of at least one uterus-related imaging record, including MRI, transvaginal ultrasonography, transabdominal ultrasonography, B-ultrasound of the whole abdomen, and CT of the pelvis; and age ≥15 years, as uterine imaging examinations are often selected by this group during an annual health examination. Exclusion criteria were the absence of a unique identification code and a positive history of hysterectomy. We separately generated two data sets for prevalence and incidence: the prevalence data set comprised women with uterine imaging data, whereas the incidence data set included women with more than two uterine imaging examinations and a negative first examination.
Height, weight, systolic blood pressure and diastolic blood pressure were measured early in the morning in the population undergoing physical examination, and body mass index (BMI) was calculated. Women usually were required to fast at least 8 hours before collecting blood from the elbow vein in the early hours of the following day to measure blood indicators such as triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), etc. Imaging examinations were performed by professional imaging doctors.
UF-associated imaging changes
We organised the reports of uterine abnormal imaging examinations with abnormalities. Reports describing ‘Potential UF’ were included directly. Additionally, from total 10 793 records of those without a direct diagnosis of UF or any other diseases, we identified 114 descriptions and sent them to experts for the confirmation of UF-associated imaging changes. The final entries of fibroid-related imaging changes were identified during two rounds of expert confirmation. The inclusion criteria for consulting experts were as follows: (1) More than 8 years of experience in the ultrasound department, (2) Intermediate or higher title, (3) Bachelor’s degree or higher education, and (4) Familiarity with UF-associated imaging changes. An electronic questionnaire was used and the principle of index screening (mean significance ≥3 points, coefficient of variation ≤0.3) was followed. A flow chart of the entry screening process is shown in online supplemental figure 1.
Supplemental material
Furthermore, based on the presence or absence of UF-associated imaging changes, the participants were assigned to the cases group or controls group, respectively.
Prevalence rate and associated factors
The prevalence was calculated with the number of individuals with UF-associated imaging changes as the numerator and the number of all individuals who met the inclusion criteria as the denominator. We calculated the prevalence rate for each year (2017–2020) and for the entire observation period. To demonstrate the trend of prevalence over age, we calculated the prevalence of each age group (grouped in 5-year intervals); then, standardised rates were calculated based on the age composition of the national female population.

R=period prevalence rate.
nc =number of prevalent cases in the population during the observation period.
N=total number of people in the observation period.
Key variables in the analysis of potential risk factors in the prevalent population (case group: participants with UF-related imaging findings; control group: women without any uterine abnormalities detected by hysterography throughout the observation period) included: BMI, blood pressure, blood lipid profile and blood glucose level. According to the Working Group on Obesity in China, BMI ≥24 kg/m2 and BMI ≥28 kg/m2 indicate overweight and obesity, respectively.31 The National Guide to Clinical Laboratory Procedures (2014 revision) was followed for the classification of dyslipidaemia, including TG, TC, LDL-C, HDL-C.32 The related determinants of prevalence were only examined among participants aged 15–49 years because UF is an oestrogen-dependent disease.
After excluding observations with >80% missing key variables, multiple imputation of complex survey data (Markov-chain Monte Carlo method in SAS PROC MI) was used to create multiple data sets. The detailed information on missing covariates was provided in online supplemental table 1. After screening for associated factors by univariate logistic regression analysis, multivariate stepwise logistic regression analysis was performed to estimate the OR and 95% CI. SAS PROC MIANALYZE was used to combine the results of the regression analysis and provide the final parametrical estimates for the regression models. Age-group-stratified analysis (≤40 years and >40 years) was performed to determine age-associated differences in associated factors for UF.
Supplemental material
UF incidence rate and potential risk factors
A retrospective cohort study was conducted to calculate the incidence rate. An incident case was defined as the first presentation of UF-associated imaging changes. The number of participants with negative results at the first of two or more uterine imaging examinations conducted more than 6 months apart was the denominator, whereas the numerator was the number of incident cases. We calculated the cumulative incidence density within the observation as well as the incidence and 95% CI for each age group (stratified at 5-year intervals).

RI 1 =incidence density, n1=number of incident cases during the observation period.
m=number of people who had two or more imaging examinations, where the first one was negative.
yi=time of observation (years), i=1, 2, …, m.
RI 2 =age-specific incidence, n2=number of incident cases in the age group.
N2=total number of potentially incident cases detected in the age group.
For incident cases, we compared the differences in baseline characteristics between incident cases and controls in the cohort. The correlation factor identification analyses were the same as those used in the associated factors of prevalence analysis.
Continuous variables were expressed as mean±SD and categorical variables as the number of cases and their percentage. One-way analysis of variance and the Student’s t-test were used to test the differences between the case and control groups. All statistical analyses were performed using Statistics Analysis System (V.9.4, Cary, North Carolina, USA). Statistical significance was set at p<0.05, using two-sided tests.
Patient and public involvement
The data of all physical examination people (2017–2020) are extracted directly from the hospital’s health check system. Patients’ personal information was hidden, and because this was a retrospective study, physical examination of people or the public were not involved in the study design or conduct or reporting of this research.
Results
The data-processing steps are shown in online supplemental figure 2. From a total of 79 746 records, we retained 46 864 records with uterine imaging findings from 33 915 women whose mean age was 42.93±12.59 years. Among 33 915 women, 8539 had at least one record of UF-associated imaging changes. Among 6168 women without records of UF-associated changes at the first imaging examination, 628 women (10.18%) presented UF-associated changes on imaging in the next 2 years. Demographic and clinical characteristics of the participants are shown in table 1. A goodness-of-fit test between the age composition of the female population in this check-up and the age composition of the female population in the seventh national census was conducted, and no statistically significant difference was found in the age composition of the population aged 15 years and older (χ2=12.04, p>0.5). The result is shown in online supplemental table 2.
Supplemental material
Demographic features and clinical characteristics of participants
Patterns of UF-associated imaging changes
As described in Methods, we identified 114 imaging descriptions and sent them to experts. In the first round of expert consultation, we sent out 12 electronic questionnaires, and 10 (83.33%) valid completed questionnaires were received; 2 experts with junior titles were excluded and 21 entries (21/114) were retained. In the second round of expert consultation, 9 questionnaires were sent out, 9 valid questionnaires were received and 17 entries were retained. The authority coefficients of the experts in the two rounds were 0.79 and 0.82, respectively. The four items corresponded to approximately 98% of the total number of positive results: ‘Potential uterine fibroids’, ‘enlarged uterus and hypoechoic nodules/masses in the uterine wall’, ‘hypoechoic nodules/masses in the uterine wall’ and ‘hypoechoic nodules/masses of the uterus’ (online supplemental table 3). In addition to those with ‘Potential UF’, many participants had ultrasound findings of ‘hypoechoic nodules/masses in the uterine wall’, and these account for approximately 30% of the case group.
Prevalence
From 2017 to 2020, 8539 cases with UF-associated imaging changes were confirmed; during the 3-year period, the crude prevalence was 25.18%, and the standardised prevalence was 21.48% when adjusted for the age composition of the national female population. The crude prevalence did not change much from 2017 to 2020 (26.17% and 28.15%, respectively). In female participants aged 15–49 years, a total of 5654 (66.21%) cases with uterine-related imaging changes were identified, with crude prevalence rates of 23.60% to 26.67% (online supplemental table 4). The prevalence significantly increased in women of reproductive age (15–49 years, value of p for trend <0.0001), peaked in the 45–49 years age group (45.38%), and then decreased to 19.89% (figure 1).
Age-group-stratified prevalence of UF-associated imaging changes in women, 2017–2020. x-axis: year. y-axis: prevalence (%).
Associated factors of UF prevalence
Patients with >80% missing rates for key variables were excluded from 8539 patients (as described in the ‘Prevalence rate and associated factors’ section), leaving 8177 patients for potential risk factor analysis. Among 8177 prevalent cases and 15 208 control subjects, compared with participants with normal BMI, those who were overweight were more likely to have UF-associated imaging changes (OR=1.34, 95% CI 1.15 to 1.56), and this result was only seen in the ≤40 years age group. High blood pressure (HBP), elevated fasting blood glucose (FBG) and elevated LDL-C levels were associated with the disease (OR=1.98, 95% CI 1.52 to 2.58, OR=2.61, 95% CI 1.36 to 4.99 and OR=1.36, 95% CI 1.13–1.63, respectively). However, in the >40 years age group, only statistically significant increases in blood pressure were observed (OR=1.24, 95% CI 1.06 to 1.46) (table 2).
Logistic regression analysis of prevalent potential risk factors for UF-associated imaging changes
Incidence
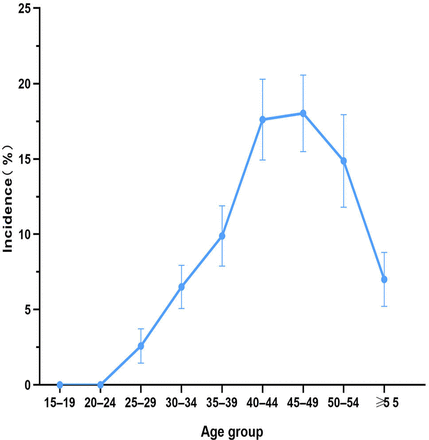
In total, 6186 women had more than two uterine imaging examinations (9413.04 woman-years). During the 3-year follow-up, 628 newly detected cases were identified, and the crude incidence density per 1000 woman-years was 63.28. The incidence rates (per 1000 women) increased with age, and were high in the 40–44 years and 45–49 years age groups in all study years (17.53 (95% CI 14.97 to 20.33) to 17.96 (95% CI 15.52 to 20.61)). The incidence in the 50–54 years age group remained high but steady at 14.73%, and significantly decreased after age 55 years (figure 2). In about 269 (42.83%) new cases, the imaging results of ‘Potential UF’ were recorded. However, 339 (53.98%) new cases had ‘Hypoechoic nodules/masses of the uterine wall’; other UF-associated imaging changes comprised a small proportion of the incident cases (table 3).
Age group-stratified incidence of UF-associated imaging changes and the composition ratio, 2017–2020
Age group-stratified incidence of UF-associated imaging changes in women. x-axis: year. y-axis: incidence (%). UF, uterine fibroid.
Potential risk factors of UF incidence
To identify potential risk factors of UF incidence, we analysed baseline data (before disease onset) of incident cases (n=491) and control participants (n=4308). In the ≤40 years age group, HBP (OR=2.16, 95% CI 1.10 to 4.24) was associated with a higher risk for UF-associated imaging changes, and being overweight (OR=1.48, 95% CI 1.03 to 2.14) potentially related to the onset of UF. However, no association was observed in the >40 years population (table 4).
Logistic regression analysis of incident potential risk factors for UF-associated imaging changes
Discussion
Using annual health examination data, the most relevant imaging signs of the disease were identified through expert consultation. We found that the prevalence of UF-associated imaging changes was 25.18% and the incidence density was 63.28 per 1000 person-years in the general Chinese population. From 2017 to 2020, the prevalence of UF-associated imaging changes fluctuated at around 38.60%–45.38%, indicating that the burden of disease of UFs is high among Chinese women, and the burden is greatest among women aged 40–54 years. Furthermore, using the baseline characteristics of the population with potential incidence, an age-stratified analysis revealed that HBP was associated with a higher risk for UF-associated imaging changes in women younger than 40 years, but not in older groups. Therefore, the results of this study provide an insight into health policy development and health resource allocation for UF early prevention.
Our results showed higher prevalence and incidence of UFs in Asians than some already published. In South Korean women (aged 15–49 years), UF prevalence is 9.0%,15 and the prevalence in all age groups was 0.96%–2.43%.16 In the USA, the incidence increased with age from 4.3% to 22.5%, whereas the incidence in Asians is 10.4 per 1000 woman-years during the past 3.67 years.33 Another study identified 1162 (6.21%) incident cases in 18 712 Asian individuals.34 In China, some studies researching other topics found 242 (10.6%) women with UF among 2277 pregnant women35 and 2204 (15.1%) cases from 14 595 retired female employees.36 However, these results were still lower than our rates in the corresponding age groups. This could be because: first, we identified UF-associated imaging changes; in addition to the inclusion of ‘Potential UF’, we also included other UF-associated imaging changes. Second, we studied the general population, as opposed to outpatients or inpatients. According to a German study, 51.4% of gynaecological diagnosed outpatients were asymptomatic,37 and this is likely to be an underestimation.38 Another reason could be that our participants were mostly employed full-time and usually lived in towns. The population with a definite unit accounted for 82.70% (47 930/57 955) of the non-missing observations. A study in China indicated that the UF prevalence was 19.6% among farmers and 26.1% in other occupations.39 However, more evidence is needed to conclusively determine the difference in the UF prevalence between rural and urban areas.40 Finally, the possibility of a higher incidence and prevalence of UFs in China, compared with other Asian populations, cannot be excluded, as presented in previous studies.
The currently recognised risk factors for UF include age.15 41–43 Our results indicate that fibroid-related imaging changes were evident with age, and both incidence and prevalence increased with age in premenopausal women whereas decreased with increasing age in postmenopausal women, and it is worth noting that the burden of disease in the 40–54 years age group is serious. Our results suggest that HBP may be a potential risk factor. Some studies support this view, and pointed out that increased blood flow and cytokines secreted by injured myometrial cells may lead to chronic destruction.44 However, some studies have shown that women with UF have a remarkably high risk for hypertension.45 46 BMI has been widely discussed in studies of risk factor for UFs, with many studies showing similar results.47 48 We found that being overweight potentially related to the onset of UF-associated imaging changes. Furthermore, women with a lower BMI (≤18.4) were less likely to have UF; however, this result was observed only in the prevalent population. A reason for the association of BMI with UFs is usually that fat can modulate hormonal and inflammatory mechanisms.49 50 In addition, we found that LDL-C and FBG were positively related to disease, and similar results were previously reported.51 52 It is unclear how LDL-C is linked to tumours; some studies have shown that oxidised LDL (ox-LDL) may act on tumour cells,42 and thereby contribute to UF occurrence.53 Insulin resistance potentially underlies pathophysiological pathways associated with obesity, diabetes, hypertension (HTN), dyslipidaemia and atherosclerosis.54 We found that overweight, HBP, higher FBG levels, and LDL abnormalities may be associated with UF-associated imaging changes, and identified HBP and overweight as potential risk factors. Variation in UF burden and factors with higher risk were noted in different age ranges. These results indicate the need for interventions based on risk stratification to help prevent or delay progression of UF. Moreover, the correlations were identified in the younger women population make it possible to be early preventive measures for women with a higher risk of UF.
The use of imaging as part of a routine health examination to identify asymptomatic individuals in the general population is feasible, relatively low-cost, and may help to understand the actual population distribution of UF. However, our study was a retrospective one, so we cannot do a more detailed analysis. To confirm the above mentioned results, further prospective studies are required.
Strengths and limitations
The strength of this study is its population-based estimates for the general women, including a large asymptomatic population. Our incidence analysis used the HBP status before the incidence of UF, suggesting HBP was associated with a higher risk for UF-associated imaging changes. Our study has limitations: Our diagnosis is merely based on the presence of disease-related imaging changes determined by expert consultation. As health check-ups aim to detect abnormalities rather than confirm a disease, UF-associated imaging changes may somewhat overestimate the actual prevalence and incidence of UF in the population, and in order to demonstrate more clearly the composition of UF-associated imaging change entries, we provide the percentage of different UF-associated imaging changes. Our study was conducted in a population that actively visited hospitals for health check-ups and is a mostly urban population, and most of our research subjects were likely to be Han Chinese. Therefore, the conclusions cannot be extrapolated to the epidemiological characteristics of UF in rural populations and other nationalities. When calculating the prevalence rate, it should be noted that our data set only encompassed a 3-month period in 2017, as that was the extent of available records within the healthcare system. However, subsequent analysis employing a goodness-of-fit test revealed that the disparity in age distribution observed during these 3 months did not demonstrate statistically significant deviation from the age distribution observed throughout the entirety of other years. And it is worth noting that there were only four obese patients in the incident case group; this may be due to the low rate of obesity among Chinese women of childbearing age.55 When the control group is patients with normal BMI values, the SE may be too large due to the small sample size of obese patients, making a false-negative result between obesity and morbidity. Finally, this analysis was based on real-world practice, which did not include certain commonly recognised factors that are related to UFs, such as behaviour, lifestyle, menstrual and reproductive history, or family history. Further, our future studies will include women from multiple sites/provinces.
Conclusions
For women of childbearing age, both incidence and prevalence increased with age and the burden of disease is highest in the 40–54 years age group, whereas there was a significant decline after menopause. The identification of HBP and overweight with higher risk for UF in younger women (age ≤40 years) presents an opportunity for implementing timely preventive interventions targeting individuals at a heightened risk of UF incidence. This holds the potential to significantly alleviate the substantial disease burden associated with UF by adopting proactive measures at an early stage.
Supplemental material
Supplemental material
Data availability statement
Data are available upon reasonable request. The anonymised participant data sets and data collection forms generated during and/or analysed (online supplemental files 1; 2) during this study are available from the corresponding author. The funder, investigators and collaborators will review and approve the proposals based on scientific merit and submit them to the Ethics Committee of Nanchong Central Hospital, the Second Clinical Medical College, North Sichuan Medical University (2021-004). After the proposal has been approved for a long period of time, data can be shared along with anonymised participant data accompanied by a data dictionary through a secure online platform after signing a data access and confidentiality agreement.
Ethics statements
Patient consent for publication
Ethics approval
All data of this study are anonymous. The protocol of this current analysis was reviewed and approved by the Ethics Committee of Nanchong Central Hospital, the Second Clinical Medical College, North Sichuan Medical University (2021–004). This was a retrospective study, and the data were collected for routine clinical purposes, not scientific research, so informed consent was waived.
Acknowledgments
The authors thank the staff of the Health Management Center of Nanchong Central Hospital for data collection and interpretation of data features for this study.
References
Footnotes
Contributors LZ: study design, writing-original draft, data acquisition, data curation, software. FX: study concept, expert support, review and editing. YH: data acquisition, data curation, software. WX, YP: expert support; writing-review and editing. KC: conceptualisation, expert support. BZ: writing-review and editing. RG: software, writing-review and editing. XS, JZ: writing-review and editing. QS: led the conceptualisation, design of the study, supervision, review and editing. QS is responsible for the overall content as guarantor.
Funding This work was supported by Grants from Health Commission of Sichuan Province, China (grant no.21PJ93); Grants from Bureau of Science & Technology Nanchong City (grant no. 16YFZJ0029) and the National Natural Science Foundation of China (No. 81872506).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.