Article Text
Abstract
Introduction Despite repeated vaccinations against SARS-CoV-2 virus, patients who are immunocompromised remain at very high risk of catching SARS-CoV-2 virus and becoming unwell. AZD7442 (Evusheld) is a long-acting monoclonal antibody treatment that has been shown in clinical trials to prevent SARS-CoV-2 infection for up to a year after a single dose. Vaccines require a healthy immune system to generate protective immunity. AZD7442 may prevent SARS-CoV-2 infection in immunocompromised individuals that may not have responded to repeated vaccinations against SARS-CoV-2 virus. Unlike vaccinations, AZD7442 reaches effective levels within the body a few hours after a single dose. The RAPID-PROTECTION trial will determine the levels of immune protection that AZD7442 offers patients at the very highest risk of SARS-CoV-2 infection and whether this protection can be further enhanced by repeated vaccination against SARS-CoV-2 virus.
Methods RAPID-PROTECTION is a multicentre, interventional and open-label adaptive platform trial that aims to recruit 350 immunocompromised participants across five UK centres. Participants will be administered AZD7442 on day 0 followed by a SARS-CoV-2 vaccination 28 days later. Participants will be randomised (1:1) to the Moderna vaccine or Pfizer/BioNTech vaccine. Participant samples will be taken at baseline and at multiple timepoints after the administration of AZD7442.
Analysis The participant samples will be analysed to measure the function and magnitude of SARS-CoV-2 specific antibody and T-cell responses at baseline and at multiple timepoints after the administration of AZD7442. The immunological effect of the study interventions will be determined by comparison of the results of immunological assessments at baseline and subsequent timepoints.
Ethics and dissemination The trial protocol was approved by the research ethics committee of the National Health Service (reference 22/HRA/0359), Health Research Authority and Health and Care Research Wales on 25 July 2022. Findings will be disseminated through peer-reviewed journals and presented at scientific conferences.
Trial registration number ISRCTN53507177.
- COVID-19
- Immunology
- Clinical Trial
- VIROLOGY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
SARS-CoV-2 infection remains a relevant health issue for immunosuppressed patients, so new clinically validated therapeutic approaches, such as those used within the RAPID-PROTECTION trial, are required, despite the availability of COVID-19 vaccinations.
Limitations of the study design include the possibility that emerging SARS-CoV-2 variants will render the AZD7442 treatment less effective.
The outcome measures collected do not necessarily have a direct correlation with infection risk.
Another limitation includes the burden of the trial visits (seven in total) on participants since each hospital visit increases their risk of contracting SARS-CoV-2 infection.
Introduction
The results from clinical trials in healthy volunteers have shown that vaccination against SARS-CoV-2 with different types of vaccines is highly effective in preventing severe disease and hospitalisation in young and old healthy adults.1–3 However, phase III vaccine efficacy studies typically excluded participants from vulnerable groups such as immunocompromised individuals, and emerging data have indicated that vaccination response in these individuals is both reduced and variable in its nature.4–10 Results from the UK OCTAVE (Observational Cohort Trial-T-cells Antibodies and Vaccine Efficacy in SARS-CoV-2) study have recently been published, which characterised the immune response to SARS-CoV-2 vaccines in 600 patients with immune-mediated inflammatory and chronic diseases; hepatic disease, end-stage kidney disease requiring haemodialysis without or with immunosuppression; solid organ cancers and haematological malignancies and patients that have undergone haemopoietic stem cell transplantation.4 The initial findings from OCTAVE have shown that patients with impaired immune systems have low or undetectable immune response to double vaccination against SARS-CoV-2 with 40% of the cohort studied mounting a low serological immune response after two SARS-CoV-2 vaccines when compared with healthy volunteers.4 The growing body of research suggests that immunocompromised patients may remain at higher early risk of SARS-CoV-2 infection despite vaccination with emerging data of SARS-CoV-2-related deaths supporting correlation between absence of detectable immunological response and efficacy in some of the above population groups.11 12 Approximately 60% of the population aged 65 or over within the UK suffer from chronic diseases, many of which are considered to lead to an additional risk of developing more severe SARS-COV-2 infection. Given the potentially increased exposure of these patient groups to infection, for example, additional hospital visits for treatment such as chemotherapy and dialysis, and the rise in emerging SARS-CoV-2 variants, alternative treatments and monitoring are required to protect these patient groups.
Immunisation against SARS-CoV-2 for the severely immunocompromised comprises primary doses followed by booster vaccines. The effectiveness of primary and booster doses is significantly reduced in certain groups of patients, such as those with solid organ transplants,13 when compared with healthy volunteers.14 Furthermore, there are relatively few data on the effectiveness of a third or a fourth SARS-CoV-2 vaccine in patients that are extremely vulnerable to SARS-CoV-2 infection. The results from OCTAVE and other published studies indicate that substantial proportions of patients with diminished immune response capacity do not respond to SARS-CoV-2 vaccines and remain at risk of SARS-CoV-2 infection despite vaccination.4 This means that, until better protection against SARS-CoV-2 can be provided to this group of patients, they will increasingly have to take measures to socially distance themselves from healthy society, which will have a negative effect on their quality of life. New clinically validated therapeutic approaches, are therefore urgently needed to protect immunosuppressed patients from SARS-CoV-2 infection.
The AZD7442 comprises the AZD8895 and AZD1061 human monoclonal antibodies (mAbs) that bind to distinct sites on the SARS-CoV-2 spike protein with nanomolar affinity.15 The SARS-CoV-2 spike protein contains the receptor-binding domain (RBD), which enables the SARS-CoV-2 virus to bind to receptors on human cells. By targeting the RBD region of the virus’s spike protein, AZD7442 blocks the virus’s attachment to human cells to prevent infection. In the PROVENT (Phase III Double-blind, Placebo-controlled Study of AZD7442 for Pre-exposure Prophylaxis of COVID-19 in Adult) study, AZD7442 was found to reduce the risk of developing symptomatic SARS-CoV-2 infection by 77% (95% CI: 46, 90), when compared with placebo.16 There were no cases of severe SARS-CoV-2 or SARS-CoV-2-related deaths in those treated with AZD7442. In the placebo arm, there were three cases of severe SARS-CoV-2, which included two deaths. AZD7442 was well tolerated, and preliminary analyses show adverse events (AEs) were similar between the placebo and AZD7442 groups.16 More than 75% of participants had comorbidities, which include conditions that have been reported to cause a reduced immune response to vaccination. Approximately 43% of participants were 60 years and over. In addition, more than 75% had baseline comorbidities and other characteristics that are associated with an increased risk for severe SARS-CoV-2 should they become infected.16 The results from PROVENT show that AZD7442 can prevent SARS-CoV-2 in symptomatic high-risk populations. However, within the PROVENT study, there were limited numbers of patients at the highest risk of developing SARS-CoV-2, and all study participants were unvaccinated against SARS-CoV-2.
SARS-CoV-2 is an RNA virus capable of rapid mutation, which can lead to amino acid changes in the spike protein that impact the efficacy of vaccines and mAb therapies. A combination mAb approach that includes two complementary mAbs, such as AZD7442, is expected to retain efficacy even if a virus variant emerges with mutations that confer resistance to one of the mAbs. Evaluation of AZD7442 on the Omicron BA.2, BA.4 and BA.5 variants has shown that AZD7442 retained neutralisation activity, with modelling assessments indicating that a higher dose of 600 mg of AZD7442 would be more effective at neutralising these variants.17–20
No data are currently available to characterise the effects of giving AZD7442 to a person who has received a course of vaccination against SARS-CoV-2. Although both booster vaccines and AZD7442 prevent SARS-CoV-2 infection and reduce the risk of life-threatening infection, they have different mechanisms of action which are complementary, and potentially immunologically synergistic. Furthermore, although the response of immunocompromised patients to SARS-CoV-2 vaccination are lower than heathy controls, immunocompromised patients will still be recommended to receive SARS-CoV-2 vaccination, even if treated with AZD7442, to maximise their protection against SARS-CoV-2. Importantly, AZD7442 reaches therapeutic levels within 4 hours of intramuscular injection, whereas SARS-CoV-2 vaccines takes up to two doses given 12 weeks apart to induce maximum protection against the infection. Therefore, giving AZD7442 could potentially induce rapid protection in patients undergoing procedures such as haematological stem cell transplantation, while their adaptive immunity recovers after their transplantation to allow them to respond to vaccination.
The RAPID-PROTECTION trial is an adaptive trial to assess the safety and immunogenicity of the AZD7442 and SARS-CoV-2 vaccines in patients who are highly immunocompromised against SARS-CoV-2 infection. The aims of the trial are to test:
That treatment with AZD7442 in combination with a SARS-CoV-2 vaccine is safe and well tolerated.
That vaccination with a SARS-CoV-2 vaccine does not reduce AZD7442 titres in humans.
That AZD7442 in combination with a SARS-CoV-2 vaccine enhances immune responses to SARS-CoV-2.
Methods
The Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines21 were used when preparing this manuscript.
Study design
RAPID-PROTECTION trial is a multicentre, interventional and open label adaptive platform trial that aims to recruit up to 350 highly immunocompromised participants across five UK centres. Patients will be stratified by cohort into four main groups: haematological malignancies, solid tumours, renal and hepatic disorders and inflammatory disorders.
Participants will receive AZD7442 on day 0 and then 28 days later a SARS-CoV-2 vaccination. Participants will be randomised (1:1) to the Moderna vaccine or Pfizer/BioNTech vaccine and the trial will be unblinded. Immunogenic response will be measured at baseline, throughout treatment and at follow-up and participants will be followed up for a total of 6 months. All Oxford patients will be tested for T cells Enzyme-linked immunosorbent spot (EliSpot) and serology (Microneutralisation assay and Meso Scale Discovery) assays to ensure matched data with stored peripheral blood mononuclear cells (PMBCs).
Eligibility
Inclusion criteria
Immunocompromised adults (≥18 years) that have one or more of the following conditions: haematological malignancies, solid tumours, renal disorders, hepatic disorders and inflammatory disorders. All participants will need to have previously completed SARS-CoV-2 vaccinations given as part of standard care at the time of enrolment, be able and willing (in the investigator’s opinion) to comply with all trial requirements and provide access to all medical records with respect to current and past medical treatments. Participants will also need a willingness to practice continuous effective contraception during the first 3 months of the trial and, if appropriate, a negative pregnancy test on the day of screening.
Exclusion criteria
A prognosis of <6 months, significant infection or other acute illness (including fever >100°F (>37.8°C)), Eastern Cooperative Oncology Group (ECOG) performance status of >2, a history of hypersensitivity, serious reactions, including anaphylaxis, serious bleeding or bruising, immune thrombocytopaenia or angioedema, or allergy likely to be exacerbated by any component of the trial interventions, a history of Guillain-Barré Syndrome, and individuals with either acute promyelocytic leukaemia, a clinically significant bleeding disorder (eg, factor deficiency, coagulopathy or platelet disorder) or capillary leak syndrome. Participants must not receive any vaccine other than the trial intervention within 30 days before and after each trial intervention (day 0 and day 28) with the exception of the seasonal influenza vaccination and non-COVID vaccinations in the case of patients receiving a haemopoietic stem cell transplant. Exclusions will also apply to those that have previously received an mAb indicated for the prevention or treatment of SARS-CoV-2, including prior receipt of AZD7442, patients who are pregnant or lactating at trial entry or planning to become pregnant within 3 months after AZD7442 administration, and those that have had blood drawn in excess of a total of 450 mL (1 unit) for any reason within 30 days prior to randomisation. Receipt of any investigational medicinal product (IMP) in the preceding 90 days or expected receipt of IMP during the period of trial follow-up or concurrent participation in another interventional trial will qualify as an exclusion unless the IMP is essential to clinical care. Patients will be excluded if they have any other significant disease, disorder or finding that may significantly increase the risk to the participant because of participation in the trial, affect the ability of the participant to participate in the trial or impair interpretation of the trial data.
Study procedures and progress
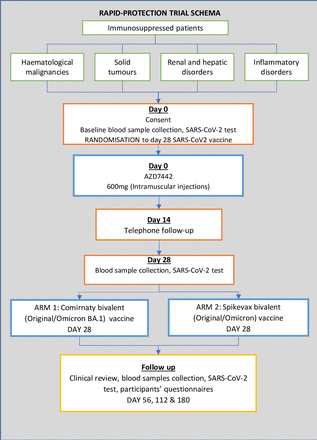
Participant samples will be taken at baseline and at additional multiple timepoints after the administration of AZD7442 for up to 12 months. The trial schema is shown in figure 1. Many factors, including the SARS-CoV-2 pandemic, resulted in a delay to the original start date of June 2022. The first participant was recruited on 15 November 2022. Current planned end date is December 2025.
RAPID-PROTECTION trial schema.
Identification and screening
Highly immunocompromised patients may be identified by the recruiting sites through their clinical treatment team and multidisciplinary team, by the research nurse from the patient notes or referred through their general practitioners (GPs) and secondary care providers to participating UK sites.
Participants will be recruited from groups known to be highly vulnerable to SARS-CoV-2, and therefore likely to benefit from AZD7442 as defined by those recommended as high priority for a third vaccine dose by the Joint Committee on Vaccination and Immunisation. Patients will be stratified into four cohorts based on their medical conditions: haematological malignancies, solid tumours, renal and hepatic disorders and inflammatory disorders. In cases where a patient qualifies for multiple cohort due to having more than one condition, they will be assigned to the cohort corresponding to their longest standing condition. If the primary cohort is full, they may enter the secondary cohort related to their other condition. Dedicated research nurses who are in direct contact with the trial delivery team and participants will ensure compliance with follow-up visits to maximise data collection. Where data cannot be collected, participants will be marked as lost to follow-up.
Informed consent
All patients will receive a full explanation on the trial including the aims of the trial, what this will involve for the participant and possible adverse effects. This conversation will be structured around the participant information sheet (PIS) and may be undertaken remotely. The individual will be given adequate time (ideally 24 hours) to read the PIS, consider the trial and the opportunity to ask questions, before being asked to sign the informed consent form—an example is given in online supplemental material. Informed consent will be taken by the local principal investigator or a trained member of the trial team delegated to do so on the site delegation log. Only when written informed consent has been obtained from the participant and they have been enrolled into the trial can they be considered a trial participant.
Supplemental material
Randomisation
Once participants are identified, consented and deemed eligible, they will be assigned a unique trial ID and randomised by the treating team via the same portal. Participants will be randomised to receive one of two boosters in a 1:1 ratio consisting of either the Pfizer/BioNTech or the Moderna vaccine.
Randomisation will be performed using random permuted blocks of sizes 2, 4 and 6, which will be chosen at random to aid in allocation concealment. The randomisation will be automated using a secure 24-hour web-based randomisation programme controlled centrally by the Centre for Trials Research (CTR) in Cardiff. The team in Cardiff will hold a manual randomisation list to be used if there are technical issues with the automated process.
Follow-up visits
Figure 2 summarises the trial procedures to be performed at each follow-up visit by the site research team. It may be necessary to perform these procedures at unscheduled timepoints, if deemed clinically necessary by the investigator. Where possible, participants’ visits should be aligned with their routine visits. Appointments that do not require clinical intervention (such as day 14) can be taken by phone where possible to limit participant’s exposure.
Schedule of interventions and assessments within the RAPID-PROTECTION trial. *Sample collected prior to trial interventions **Lateral flow testing for SARS-CoV-2 infection to be performed for participants at the Oxford University Hospitals National Health Service site only. If the lateral flow test is positive for SARS-CoV-2 infection, a nasal PCR swab will be taken to be sent for testing. FBC, full blood count; LFT, liver function test; U&E, urea and electrolytes; PROMIS 10, Patient-Reported Outcomes Measurement Information System Global Health; EQ-5D-5L, EuroQol 5D-5L.
Additional vaccines may affect the results of the study and not give an accurate picture of the protection provided by Evusheld. Unless the study team specifically advise otherwise, participants are not permitted to receive any of the approved National Health Service (NHS) SARS-CoV-2 vaccinations for 3 months after their SARS-CoV-2 vaccination within the study.
Sample management
Sites will be provided with a translational sample collection kit. Up to 50 mL of blood will be collected from participants at the following timepoints: baseline (predose), and 28, 56, 112 and 180 days post AZD7442 administration. The blood samples will be processed to serums and frozen onsite. The CTR will arrange regular batch collections of serum samples on dry ice to the relevant laboratory. Samples may be stored by laboratories approved by the sponsor, so that immunogenic response measurements can be performed on all samples at the same time. The Oxford University Hospitals NHS site will be asked to provide fresh blood samples for additional analysis. Full details on sample collection and management can be found in the RAPID-PROTECTION laboratory manual.
Asymptomatic lateral flow tests for SARS-CoV-2 infection will be performed for participants at the Oxford University Hospitals NHS site routinely on days 0, 28, 56, 112 and 180. If the lateral flow test is positive for SARS-CoV-2 infection, a nasal PCR swab will be taken to be sent for testing.
Participants at all sites who self-report testing positive to SARS-CoV-2 throughout the trial will also be asked to send a nasal PCR swab for testing.
Data collection
All data collection will be by electronic data capture using a bespoke database developed by the CTR (Macro V.4.9.1, Ennov, Oxford, UK) and hosted by Cardiff University secure servers. It is encrypted and accessed by individual username and password and complies with the General Data Protection Regulation standards. A member of the research team at the participating UK sites will undertake the data collection. Participants who consent to the study will have information collected from the date of randomisation until the completion of all follow-up visits (see figures 1 and 2).
The participant’s NHS number will be collected to allow linkage with national data registries such as NHS Digital, Public Health England, Health and Care Research Wales (HCRW) and the Information Services Division (part of NHS Scotland) or the electronic Data Research and Innovation Service. Data linkage will allow for long-term follow-up data to be collected, and it will provide a more complete profile of the participants’ health and disease without increased data collection burden to the NHS.
Withdrawal
Participants have the right to withdraw consent for participation in any aspect of the trial at any time. Participants may request that all previously collected samples are destroyed to prevent use in future research, though it may not be possible to retrieve samples already donated for future research (eg, if samples have been anonymised). If a participant wishes to fully withdraw from the trial, they will need to be seen one last time for assessments and tests.
Safety and pharmacovigilance
AEs will be reported within the trial as per the standard definition. For the purpose of the trial, the following events will also be considered AEs of special interests: grade≥3 anaphylaxis and other serious hypersensitivity reactions, including immune complex disease (defined by regulatory criteria of seriousness and Medical Dictionary for Regulatory Activities (MedDRA) Standardized MedDRA Queries Hypersensitivity (Narrow)), cardiac events (ie, cardiac ischaemia, cardiac failure and thrombotic events), grade≥3 injection site reactions (defined by MedDRA High-Level Term Injection site reactions) and SARS-CoV-2 infections that are life-threatening, requiring hospitalisation or result in death within start of treatment and 28 days following vaccine booster.
The trial population comprises immunocompromised participants that have pre-existing conditions. Pre-existing conditions and hospitalisations as a result of these should only be reported if they meet the definitions for a serious adverse event (SAE) and if the condition worsens by at least one Common Terminology Criteria for Adverse Events (CTCAE) grade.
Data management
Details of data management procedures, such as checking for missing, illegible or unusual value (range checks), will be specified in the RAPID-PROTECTION data management plan. Details of monitoring procedures will be specified in the RAPID-PROTECTION monitoring plan.
Statistical analysis
Sample size
The trial aims to recruit a total of 350 patients with immunosuppressive conditions. Samples sizes required for analysis of the various cohorts and outcomes have been based on SARS-CoV-2 immunoglobulin (Ig) responses taken from the analysis of the PROVENT trial and other similar trials.
Participants will be stratified into subcohorts as summarised in figure 3. This will be an adaptive trial that will extend recruitment in any subcohort where more information is required in order to come to a reliable estimator. The immunological assessments will be analysed to allow cohorts to be expanded in case of poor immunogenicity to SARS-COV-2 or closed if good immunogenicity against SARS-COV-2 is seen. Recruitment of patients from each group will be limited to ensure the trial captures a representative group of patients from all major immunosuppressive groups most at risk of SARS-CoV-2 mortality. The group sizes will be calculated based on the expected heterogeneity of the group and the degree of variability in the general population. This may include subdividing groups by disease type to gain more homogenous responses.
The cohorts and further subgrouping of immunocompromised participants within the RAPID-PROTECTION trial. JCVI, Joint Committee on Vaccination and Immunisation.
To account for an anticipated 20% attrition rate, we plan to recruit a total of 350 participants, with an expected enrolment rate of 70 participants per cohort and this is anticipated to take 3 months. Our objective is to compare cohorts 1, 2, 3 and 4 with healthy volunteers, aiming for 90% statistical power at a 5% significance level in the log domain with a SD of 1. To achieve this, we require a minimum of 70 patients in each cohort.
This power calculation is based on our expectation that Ig levels in the patient cohorts will exhibit greater variance compared with healthy volunteers. Consequently, we have powered the study to enable this comparison within each of the four cohorts, totalling 280 participants. Accounting for a 20% potential loss to follow-up, our recruitment target is set at 350 patients, distributed evenly across the selected cohorts.
To maximise cohort-level heterogeneity and enhance branching decisions, we will strive to achieve balanced recruitment within subcohorts of each main cohort, as depicted in figure 3.
Primary outcomes
Most primary outcome measures are taken directly or derived from bioanalytical assays. The following are designated primary outcomes:
Pharmacokinetics (PK) of serum concentrations of AZD7442.
Effect of a SARS-CoV-2 vaccination on serum concentration titres of AZD7442.
Humoral and cellular response measured by anti-SARS-CoV-2, anti-S-RBD total Ig and antigen specific T cells recognising the SARS-CoV-2 S antigen.
Levels of neutralising antibodies to SARS-CoV-2.
AEs and SAEs.
Secondary outcomes
The following are secondary outcomes:
To assess if different SARS-CoV-2 vaccines will preferentially enhance humoral and/or T-cell responses in immune suppressed patients receiving AZD7442.
The incidence of SARS-CoV-2 infection in trial participants.
The severity of SARS-CoV-2 infection in trial participants.
The incidence of participants who have an increase in SARS-CoV-2 nucleocapsid antibodies (from negative/low at baseline to low/high at any time post baseline).
Sequencing of confirmed SARS-CoV-2 infections to identify SARS-CoV-2 variants and potential AZD7442 escape variants.
To assess the behaviour of the trial participants before and after trial treatment.
Analysis
The analysis of cohorts 1, 2, 3 and 4 will commence on obtaining 280 completed datasets at the 28-day mark. If the variance in Ig levels is shown to be significantly different to age and gender matched controls or more variable, then the analysis will progress to subcohorts A, B, C and D individually and recruitment targets re-estimated.
Further details of the statistical analysis will be specified in the RAPID-PROTECTION statistical analysis plan. In general, a treatment policy approach to estimands will be used to deal with intercurrent events.
Pharmacokinetics (PK)
AZD7442 serum concentrations (ng/mL) will be measured at baseline and days 28, 56, 112 and 180. Serum concentrations and titres of AZD7442 will be calculated at each time point and summarised using descriptive statistics (mean, medium, SD, IQR and range). Means and two-sided 95% CI will be calculated and plotted over time, overall and for each cohort. PK parameters Tmax and Cmax will be calculated and summarised using means and standard deviation (Cmax) or medians and IQR (Tmax). Comparisons of Cmax between cohorts will be made using analysis of covariance (ANCOVA) models adjusted for age and gender. Mann-Whitney U tests will be used to compare Tmax between cohorts, age groups and gender.
Immunogenicity
Immunogenicity measurements are:
Serology analysis consisting of measuring the anti-SARS-CoV-2 anti-S-RBD total Ig antibody concentration at baseline and days 28, 56, 112 and 180 post-treatment with AZD7442. The concentrations are assessed using the Roche assay and measured as ELISA units per millilitre.
Assessment of antigen specific T cells recognising the SARS-CoV-2 S antigen, specifically the ex vivo interferon gamma EliSpot assay. The outcome is measured as spot forming cells per million peripheral blood mononuclear cells. These will be measured at baseline and days 28, 56, 112 and 180 post treatment with AZD7442 in up to 80 selected Oxford patients.
Neutralising antibodies will be measured at baseline, day 28, day 56 and one other timepoint (not yet specified) post-administration of AZD7442. A specific neutralising antibody assay will be used to measure the percentage neutralisation (IC50).
Immunogenicity data will be presented as means or medians and SD or IQR/range at each timepoint overall and for each cohort and subcohort. Comparisons of anti-SARS-CoV-2 anti-S-RBD total Ig plus monoclonal antibody (PK assay) will be made with age and gender matched ‘healthy controls’ from the PROVENT III trial, overall and for each cohort. Group data will be requested from the PROVENT trial. Values will be log-transformed and mean and SD calculated for the same groups at each timepoint. The PROVENT data will be used to calculate the overall SD of a population with the same age/sex distribution as each of our cohorts at each timepoint. The mean and SD and its 95% CI will be calculated for each cohort for comparison with the PROVENT data at each timepoint.
This method will be used to analyse the immunogenicity outcomes at 28 days following AZD7442 administration, and 56 days following AZD7442 administration once patients have also had their vaccine booster. Comparisons will be made between each of the vaccines using an ANCOVA model with baseline value as a covariate. An exploratory analysis will be conducted to examine differences between the vaccine types and patient cohorts.
Adverse events (AEs) and serious adverse events (SAEs)
AEs and their severity, assessed using CTCAE V.5.0, will be evaluated at baseline, and on days 14, 28 and 56. For SAEs, assessments will include severity (using CTCAE V.5.0), expectedness, seriousness and their relationship to each cohort and subcohort at baseline. Additionally, all AEs and SAEs will be summarised by their type of toxicity, using the MedDRA System Organ Class, preferred term at baseline, and for the most severe reports within each cohort and across all cohorts, up to day 28 and day 56.
The number percentage and 95% CI of patients experiencing each AE/SAE, categorised by severity will be tabulated. 95% CI will be calculated using the Clopper-Pearson method as it is likely AEs will be relatively uncommon. Numbers (percentages) of SAEs, serious adverse reactions (SARs) and suspected unexpected SARs will be compared by cohort, subcohort and overall.
Dynamics of nucleocapsid antibodies over time in patients during the course of the study
The incidence of participants who have a post-treatment increase (negative/low at baseline to positive at any time post baseline) in SARS-CoV-2 nucleocapsid antibodies (anti-SARS-CoV-2 nucleocapsid total IgG (results provided as cut-off index) will be assessed at baseline and days 28, 56, 112 and 180. Infection naïve participants at study entry who become positive during the course of the study (negative to low/high), and those participants with low nucleocapsid antibodies at study entry that become high, will be presented by cohort, subcohort and overall.
SARS-CoV-2 infections
Incidence of SARS-CoV-2 infection will be ascertained by lateral flow test and (if positive) PCR swab test and analysis will consist of a descriptive analysis summarised by incidence rates and 95% CI; this will include data on SARS-CoV-2 strain. This will be performed overall and over specified timeperiods. Comparison of incidence rates between cohorts will be performed using Poisson regression and summarised as incidence rate ratios, risk differences, 95% CI and p values.
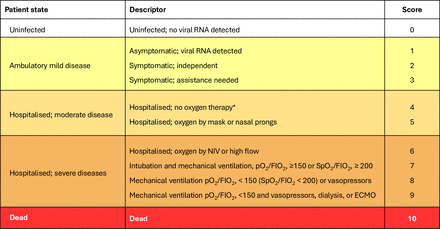
The assessment of SARS-CoV-2 severity will be measured by the WHO Clinical Progression Scale (figure 4). The frequency and percentage of patients in each score category will be tabulated overall and in each study cohort.
WHO Clinical Progression Scale (reproduced from https://www.who.int/23). ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; NIV, non-invasive ventilation; pO2, partial pressure of oxygen; SpO2, oxygen saturation. *If hospitalised for isolation only, record status as for ambulatory patient.
For participants who are PCR swab test positive and confirmed SARS-CoV-2 cases, genomic RNA sequencing will be performed to identify the SARS-CoV-2 escape variants. DNA analysis of genetic polymorphisms potentially relevant to vaccine immunogenicity and/or SARS-CoV-2 pathogenesis or SARS-CoV-2 therapeutics and gene expression studies among others may be performed at the discretion of the investigators.
Patient questionnaires
Patient behaviour before and after treatment will be measured using a standard questionnaire consisting of the following instruments household composition (only at baseline), COVID-19 Risk Behaviour Changes, EQ5D-DL Quality of Life and PROMIS V.1.2.
Household composition at baseline will be summarised using medians and IQR and presented in baseline tables.
Patient COVID-19 risk behaviour questionnaires will be summarised using frequencies and proportions over time. PROMIS Scale V.1.2—Global Health will be scored as described in the scoring manual.22 For continuous measures, for example, EuroQol Visual Analogue Scale, responses will be summarised using means and SD or medians and IQR dependent on the distribution of the baseline data. Differences in responses for questions between cohorts will be assessed using the Wilcoxon-Mann-Whitney test. Changes in responses over time will be examined using multilevel models accounting for the longitudinal nature of the data.
Interim analysis
The data will first be reviewed by the independent data monitoring committee (IDMC) when one-third of patients (116) have been recruited and data at 28 days post AZD7442 administration has been returned. This will consist of recruitment, data collection and safety data. Subsequent meetings will be held at the end of recruitment and if a potential adaptation to extend recruitment in any subcohort where more information is required in order to come to a reliable estimator.
Patient and public involvement (PPI)
The study has been reviewed by two independent PPI members who have been included in the trial design, review of the study and all patient facing documents. Both PPI members are members of the trial management group (TMG) meaning that they remain key to contributing to the trial as it progresses by making judgements based on their individual conditions or personal experiences. Both PPI members have made active contributions throughout the life of the trial and continue to provide a different perspective to the other researchers and clinicians that are involved in the trial. Should the trial undergo adaptation, the PPI members will remain critical to advising in any changes to the trial design, such as by providing insight into relevance and burden of the trial on participants. Furthermore, a patient representative is included as a member within the trial steering committee (TSC).
Trial management
The trial is being sponsored by Cardiff University with responsibilities delegated to the CTR.
Trial management group
The TMG will be responsible for the day-to-day running of the trial and will meet at least once every 3 weeks in the first instance. The frequency of this will be reduced following the trial opening. The TMG members will include at least the Chief Investigator, other active trial investigators, CTR trial statistician and CTR trial manager. It will also include at least one consumer representative. TMG members will be required to sign up to the remit and conditions as set out in the TMG charter.
Trial steering committee (TSC) and independent data monitoring committee (IDMC)
An independent TSC consisting of an independent chairperson, two independent members and a patient representative will provide oversight of the RAPID-PROTECTION trial. There will also be a separate IDMC consisting of at least two clinicians (not entering patients into the trial) and an independent statistician. The IDMC will provide oversight of all matters relating to patient safety and data quality and recommend continuing or stopping the trial depending on the results of the interim analysis. Members will be required to sign up to the remit and conditions as set out in the TSC and IDMC charters and will meet at least annually.
Ethics and dissemination
Ethics approvals and consent
The trial was approved by the research ethics committee of the NHS (London—Fulham REC, reference 22/HRA/0359), Health Research Authority and HCRW on 25 July 2022. The following substantial amendments were made to the trial and were communicated to all trial sites: amendment 1 (23 September 2022); amendment 2 (04 November 2022); amendment 4 (20 July 2023); amendment 5 (30 August 2023). The current approved protocol version is V.5.0, dated on 09 May 2023.
Dissemination plan
Findings will be disseminated through peer-reviewed journals and presented at scientific conferences. All publications and presentations relating to the trial will be authorised by the TMG in accordance with the RAPID-PROTECTION publication policy. Data from all sites will be analysed together and published as soon as possible. The main trial results will be published in the name of the trial in a peer-reviewed journal on behalf of all collaborators.
All participants will receive an individual participant results letter that will include their antibody test results for their baseline and 28 and 180 days post AZD7442 administration samples. This will indicate how well their immune system has previously responded against the SARS-CoV-2 virus.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to thank the research nurse and clinicians for their support during the trial. They would also like to acknowledge the contribution of the trial steering committee members, namely Professor Richard Wilson, Professor Helen Parry, Dr Michelle Willicombe, Dr Elizabeth George, Professor Thushan de Silva, Julie Hepburn and Jan Davies. The authors would also like to acknowledge the contribution of independent data monitoring committee members, namely, Dr Thomas Newsom-Davis, Dr Ian Rowe, Professor Graeme MacLennan and Dr Taryn Youngstein.
References
Footnotes
X @PEACH_Study1, @toomuchaltitude, @emma_tj1
Contributors MT is the chief investigators of this trial. MT, along with EB, RR, KE, AH, ET-J, LSN, KH, CP, RA, CHO, KW, SK, SG and EC led the implementation of the protocol. LSN and ET-J were jointly responsible for the overall delivery of the trial from Centre for Trials Research. MV is the trial manager and JE is the senior trial manager who coordinate the operational delivery of the trial protocol and recruitment. CP is the trial statistician. DH is the senior data manager and CB is the data manager. LR is the senior data and analytics manager. MV, JE, ET-J, LSN, CP, KH, RA, DH, LR, CB, CHO, KW, SK, SG, EC, AH, RT, SP, VD, PC, MT and EB are all trial management group members. All authors listed provided critical review and final approval of the manuscript. MT is the guarantor.
Funding The RAPID-PROTECTION trial is being funded by AstraZeneca (funder reference: ESR-21-21549) and is part of the National Institute for Health and Care Research Portfolio. The Centre for Trials Research receives infrastructure funding from Health and Care Research Wales.
Disclaimer The views expressed are those of the authors and not necessarily those of the funder, NHS, the NIHR or the Department of Health and Social Care. Neither the Sponsor nor the Funder had any role in the study design; collection, management, analysis and interpretation of data; writing of this manuscript or in the decision to submit this manuscript for publication.
Competing interests MT has declared receiving fees relating to Evusheld and breast cancer from AstraZeneca, Pfizer, Novartis, Lilly, Janssen, Roche, Vaccitech, BMS, Oxford Vacmedix, Ipsen, Chugai, MSD, Sanofi, BMS, Genomic Health, Astellas, Esai, Everything Genetic, Chugai, Bayer, X and Seagen; and Immunocore for participation on a Data Safety Monitoring Board. EC has declared receiving research grants or contracts from Bio-Cancer, Biogen, Pfizer and Sanofi; consultancy fees for Abbvie, Biogen, Bristol Myers Squibb, Eli Lilly, Fresenius Kabi, Gilead, Janssen, Sanofi-Genzyme and UCB; receiving payments from Abbvie, Chugai Pharma, Eli Lilly, Fresenius Kabi, Galapagos, Pfizer, UCB, Janssen and Sanofi.
Patient and public involvement Patients and/or the public were involved in the design or conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.