Article Text
Abstract
Introduction Yeast beta-glucan (YBG) are recognised for enhancing the immune system by activating macrophages, a key defence mechanism. Given the global prevalence and impact of upper respiratory tract infections (URTIs) on productivity and healthcare costs, YBG has shown promise as a potential therapeutic and preventive strategy for recurrent respiratory tract infections. However, little is known regarding the efficacy of YBG at lower dosages in relation to URTI, fatigue, immune response and uncertainties of how they affect the gut microbiota composition.
Methods and analysis This 12-week randomised, double-blinded, placebo control, parallel-group clinical trial aims to evaluate the efficacy of YBG 1,3/1,6 on respiratory tract infection, fatigue, immune markers and gut health among adults with moderate stress. The study involves 198 adults aged 18–59 years with moderate stress levels as assessed using Perceived Stress Scale 10 (score 14–26) and Patient Health Questionnaire 9 (score ≥9); and had symptoms of common colds for the past 6 months as assessed using Jackson Cold Scale. These participants will be randomised into three groups, receiving YBG 1,3/1,6 at either 120 mg, 204 mg or a placebo. The outcomes measures include respiratory infection symptoms, fatigue, mood state and quality of life assessed using Wisconsin Upper Respiratory Symptoms Scale, Multidimensional Fatigue Inventory, Profile of Mood State and Short Form 36 Health Survey Questionnaire, respectively. In addition, full blood analysis and assessment of immune, inflammatory and oxidative stress biomarkers will be taken. Secondary outcome includes gut microbiota analysis using stool samples via 16S rRNA sequencing.
Ethics and dissemination The research protocol of the study was reviewed and approved by the Research Ethics Committee of Universiti Kebangsaan Malaysia (UKM/PPI/111/8/JEP-2023–211). The findings will be disseminated to participants, healthcare professionals and researchers via conference presentations and peer-reviewed publications.
Trial registration number ISRCTN48336189
- COMPLEMENTARY MEDICINE
- Clinical Trial
- Immunology
- NUTRITION & DIETETICS
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is a randomised, double-blinded, placebo-controlled, parallel-group study with a comprehensive range of immunity markers and well-designed human intervention trials to evaluate the mechanism of action behind the effect of yeast beta-glucan (YBG) on immune function in humans.
The study assesses YBG’s immunomodulatory effects in moderate-stress adults using two lower dosages of 120 mg and 204 mg, compared with the command dosage of 250 mg, to be administrated for 90 days.
Analyses of the gut microbiota composition in faecal samples by 16S ribosomal RNA sequencing.
The primary constraint in this 12-week study revolves around loss to follow-up, missing data points and the compliance of participants which collectively challenge the validity of the reported results during the data analysis phase.
Recruiting eligible study participants is constrained by hesitancy or discomfort related to providing stool samples.
Introduction
Background and rationale
Globally, respiratory tract infections have recently ranked among the top five health issues, affecting 153.05 million people and significantly contributing to morbidity and mortality.1 Upper respiratory tract infections (URTIs) are particularly prevalent in primary care settings, including Malaysia, where respiratory conditions account for 26.8%–37.2% of all health problems.2 3 Although URTIs are often self-limiting, they can impair quality of life and productivity,4 and may sometimes lead to complications, such as pneumonia and otitis media, adding to the financial burden of healthcare.5 6
Fatigue, often driven by sleep deprivation and workplace stress,7 8 is linked to a higher risk of URTIs and negatively impacts health and productivity across various professions.9–12 When individuals experience fatigue, their immune systems may become compromised, making them more susceptible to infections. Fatigue can arise from juggling multiple jobs, prolonged multitasking and navigating psychosocial challenges in the workplace, all of which can lead to increased stress levels and decreased overall well-being. Consequently, the combination of physical exhaustion and psychological strain can impair the body’s ability to resist pathogens, heightening the likelihood of developing URTIs.13 Thus, addressing the root causes of fatigue is crucial for reducing the risk of infections and improving workplace productivity.
A well-functioning and resilient immune system is crucial for staying healthy.14 The gut microbiota, including bacterial phyla such as Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria known for its impact on metabolic functions and immune responses in the human body,15 is influenced by diet, as demonstrated in a recent study.16 Certain gut bacteria such as Lactobacillus and Bifidobacterium may produce metabolites that can positively affect the respiratory system and immune response, indirectly influencing URTI susceptibility.17 Furthermore, the gut and respiratory tract maintain bidirectional communication through the gut-lung axis, enabling interaction between the gut microbiota and respiratory health.18 Maintaining proper nutrition and a healthy lifestyle is crucial for strengthening the immune system. Baker’s yeast beta-glucan (YBG), specifically the beta 1,3/1,6-glucan derived from Saccharomyces cerevisiae, is a polysaccharide known for its immune-modulating properties.19 It has shown wide-ranging significance due to its anti-cancer,20 anti-inflammatory21 and immunomodulatory effects.22 Beta-glucan from yeast and fungi (1,3/1,6) linkage with side-chain branches attached to the backbone, such as S. cerevisiae and various medicinal mushrooms like Reishi (Ganoderma lucidum) and Shiitake (Lentinula edodes) has been extensively studied for its immune-enhancing effects.23 They are also recognised for their prebiotic effects, meaning they can serve as food for beneficial bacteria in the gut.24 For example, a study by Morales et al25 demonstrated that beta-glucan derived from Lentinus edodes, when administered over 8-week period, significantly modulated the composition of colonic microbiota as compared with the placebo group.25 Thus, how these properties modulate biological functions in terms of molecular mechanisms is currently being intensively studied.
Most of the research on YBG26–28 primarily centres around high dosages ranging from 250 mg to 1000 mg; typically administered to healthy adult populations. For example, a placebo-controlled trial among 202 marathon runners involving 91 days of administration of 250 mg of S. cerevisiae reported an improvement in URTI symptom severity.29 Similarly, a study conducted over 90 days among 100 healthy adults using the same dosage also showed a significant reduction in URTI symptoms.30 Although high dosages yield significant results, research findings suggest that low dosages also lead to improvements and similar effects. This is supported by a prior study on probiotic supplementation, which found that administering lower dosage for 28 days resulted in effects comparable to those of higher dosage, with individual probiotic strains detected in faeces in proportion to the dose, indicating that both low and high dosages were effective and safe for consumption.31 Likewise, Wu et al found that even at lower doses (200 mg), a combination of beta-glucan from S. cerevisiae, Ganoderma lucidum and Lentinus edodes effectively stimulated immune cells, enhancing immune activity and reducing the severity of upper respiratory infections.32 Besides, using lower doses of beta-glucan in clinical trials help to minimise the risk of side effects, such as gastrointestinal discomfort including bloating and cramping.33
Nevertheless, there is a paucity of data for scientific evidence exploring the efficacy of lower dosages for YBG (below 250 mg) and their potential effects on various health outcomes, including fatigue and changes in gut microbiota.34 35 Understanding the threshold at which beta-glucan provides benefits will help in recommending appropriate and cost-effective dosages for individuals. Thus, this current study aims to be the first clinical trial to investigate the efficacy of YBG 1,3/1,6 at a lower dosage on fatigue, respiratory tract infections and its impact on gut health. Therefore, this study is essential to address the current research gap and potentially provide new insights into low-dose YBG supplementation as an affordable and practical approach for enhancing immunity, reducing fatigue and supporting gut health. A deeper understanding of the gut microbiota’s role in respiratory diseases will help inform future therapeutic approaches.
Research objectives
The research primary objective of this RCT intervention study is to investigate the effects of YBG 1,3/1,6 with placebo on URTI symptoms, fatigue level, mood state, quality of life and immune response among moderate-stress adults. Gut microbiota will be assessed as the secondary objective.
Primary endpoint
The primary objective of this RCT intervention study is to investigate the effects of YBG by comparing the effects of YBG with placebo on the URTI episodes, fatigue, mood state, quality of life, blood immune markers, inflammatory and oxidative stress biomarkers on moderate stress adults.
Secondary endpoint
The gut bacterial diversity (α diversity, β diversity, comparative analysis) and predicted functional analysis will be assessed as the secondary objectives.
Other parameters
Anthropometric measurement, full blood profile, 3 day food record will be evaluated pre-intervention and post-intervention.
Hypothesis
We hypothesised that the efficacy of YBG potentially benefits respiratory infection, fatigue, mood state, quality of life, immune markers and gut health among moderate-stress adults.
Methodology & analysis
Study design
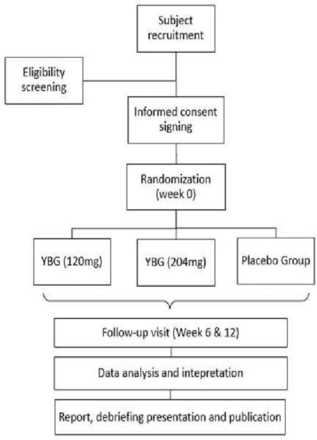
The study design is a three-armed randomised, double-blind (study participant and the investigator), placebo-controlled, 12 weeks parallel-group clinical trial. A brief research flow chart is shown below in figure 1. We used the SPIRIT checklist (online supplemental appendix 5) as a guideline while designing the study protocol.36
Supplemental material
Research flowchart. YBG, yeast beta-glucan.
Sample size calculation
Calculation of sample size is determined by the Randomised Control Trials formula proposed by Chan and Chan.37 The sample size calculation was performed based on the primary endpoint of the study, which was infectivity. The result from a previous study29 investigated whether YBG is useful in reducing incidences of URTI in marathon runners.

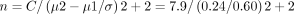
n=51,where n=sample size

μ1 – μ2=mean difference between the intervention (0.38±0.60) and control group (0.52±0.71) after the 91 days intervention, mean of total number of URTI episodes
σ=the SD of the intervention group29
C=constant: 7.9 (80% power and 95% CI)
With consideration that the dropout rate of subjects is 30%, the sample size would be 51+11 = 66 subjects per arm.
Total sample size (3 arms) = 198 participants
To achieve the targeted sample size, approximately 500 individuals will be screened for inclusion and exclusion criteria.
Participant recruitment and screening
Purposive sampling will be conducted around Klang Valley and the official study site will be located at Respiratory Clinic, Hospital Canselor Tuanku Muhriz and Centre for Healthy Ageing and Wellness, the National University of Malaysia. Advertisements on free health check-ups related to the screening will be held around the Klang Valley area to recruit potential participants of the study. The potential participants will be screened for psychological stress and mental health status using the perceived stress scale38 and Patient Health Questionnaire 9.39 A history of URTI of at least one infectivity for the past 6 months will be assessed using Jackson Cold Scale.40 The eligible participants will be provided with a participant information sheet and written informed consent forms (online supplemental appendix 1, 2) before their participation. Recruitment will continue throughout the study until the required number of participants is achieved. This study will be carried out following the Helsinki Declaration of 1975, as revised in 1989, which states that no vulnerable participants should be recruited. The study is currently in the recruitment phase. The screening process will start in January 2024.
Eligibility criteria (inclusion and exclusion criteria)
Participants will be recruited into the study based on the inclusion and exclusion criteria (table 1).
Inclusion and exclusion criteria
Randomisation and intervention
A 12 weeks, randomised, double-blinded study will be carried out to compare the effects of YBG supplement against placebo. Eligible participants will be selected and randomly allocated to one of the three groups (120 mg YBG, 204 YBG and 0 mg YBG placebo) using computer-generated random numbers obtained through online sample randomiser software (graphpad.com). According to their order of recruitment, each participant will be assigned a study participation ID numbered from 001 to 198, and it will remain unchanged throughout the study. All the eligible participants will be randomised to receive one sachet daily for the study duration of 12 weeks.
Preparation of yeast beta-glucan supplement and placebo
The supplement is made into a white granular powder with mixed berries flavours, sealed in an aluminium foil sachet. Each 2 g sachet contains either 120 mg or 204 mg of YBG, or a placebo (table 2). Participants will be directed to take one sachet daily, either in the morning or evening, either consumed directly or by mixing the powder with water during a meal. Besides, the participants will be reminded to store the supplement in a dry place and keep it away from direct sunlight or below 25°C. Regular phone messages will be sent to remind them to adhere to the instructions for using the interventional products. This strategy will also facilitate continuous monitoring and prompt response to any potential side effects.
Specification of yeast beta-glucan and placebo
To maintain the blinding procedure, the YBG supplements and placebos will be labelled using a coded system. Only the manufacturer and packager will know the coding for either YBG-A, YBG-B or YBG-C labelled on the sachets. The three groups share the same look, fragrance, texture and taste. The codes will remain undisclosed until the completion of the analysis to ensure the integrity of the blinding.
Clinical assessment
After the recruitment, participants who have consented will be directed to the study site for baseline assessment. Participant’s sociodemographic data, medical history and concomitant medication history will be recorded at the baseline for safety purposes. Anthropometric and body composition measurements will be recorded. Anthropometric measurements will be conducted based on the procedures described by Gibson41 and the WHO.42 These included body weight, height, body composition (muscle mass, fat mass and fat percentage), waist circumference and hip circumference. Weight and body composition will be measured using bioelectrical impedance analysis with a body composition analyser (Model; SC-330, TANITA Japan). The standing height of the participants will be measured using a stadiometer to the nearest 0.1 cm, and body mass index (BMI) will then be calculated. Waist and hip circumference will be measured using a soft measuring tape to the nearest 0.1 cm. The waist-hip ratio will be calculated by dividing the waist by the hip circumference. Muscle mass (kg), fat mass (kg) and fat percentage (%) will be extracted and copied into the database. All anthropometry measurements will be conducted in duplicates, and mean readings will be calculated using a standard method.43 Besides that, vital signs of participants, including blood pressure will be measured using an electronic blood pressure machine (Omron Kyoto, Japan) during all visits.
A trained dietitian or nutritionist will assess the dietary intake of the participants using a 3 day food record,44 along with prebiotics and probiotics food checklist intake. The 3 day food record will document the food and drinks consumed by the participants on 2 week days and 1 day of the weekend each week. Participants will be asked to provide as much detail as possible on the meals taken, such as type and amount consumed, method of preparation, food brands, sauce and ingredients used. Participants will be shown pictures of various household measurements based on the Atlas of Food Exchanges and Portion Sizes45 to help them estimate the portion size. Any supplements such as vitamins or minerals consumed will also be recorded. In addition, a prebiotics and probiotics food checklist questionnaire will also be used to identify the types, frequency, and quantity of prebiotics and probiotics content food consumption among participants. Computer-based analysis of mean caloric and nutrient intake will be performed using the Nutritionist Pro software (Axxya Systems, Stafford, TX) using Malaysian food database.46
The primary outcomes of the study which are the participant’s URTI episodes (occurrence, severity and duration), fatigue, mood state and quality of life, will be measured using the Wisconsin Upper Respiratory Symptom Survey21,47 Multidimensional Fatigue Inventory,48 Profile of Mood State49 and Short Form Health Questionnaire 3650 questionnaire (online supplemental appendix 3), respectively. Participants will be required to fast for 10 hours before peripheral venous blood withdrawal. A qualified phlebotomist will collect 20 mL of fasting venous blood to measure full blood count, HbA1c, fasting plasma glucose, renal function test, liver function test and lipid profile. Blood immune markers including cytokines (TNF-α and IFN-γ), interleukin (IL-6, IL 8, IL-10), inflammatory biomarkers (inducible nitric oxide synthase (iNOS), cyclooxygenase [Cox2]) and oxidative stress biomarkers (malondialdehyde (MDA), lipid peroxidation (LPO)) will be analysed at baseline and week 12 (online supplemental appendix 3). Blood will be transferred into their respective types of vacutainers and delivered to a certified diagnostic laboratory (Gnosis Laboratories (M) Sdn Bhd, Selangor, Malaysia; 5 77 284 A) for analysis. The blood test results will be mailed or given to the participant during the next follow-up. Contact details are provided along with the results to enable the participant to contact the investigators if they have any inquiries.
Stool sample collection
The secondary outcome of the current study is to determine the changes in gut health, focusing on microbiota diversity and functional analysis prediction resulting from YBG intervention. For this purpose, each of the three groups will be assigned 50 participants based on their willingness to collect stool samples. Stool sample collection will be conducted at baseline and 12 weeks post-intervention. The participants will be provided with a stool collection kit to take home and detailed instructions on how to collect the stool sample. This stool collection kit contains: (1) a Bunny Wipe with a DNA/RNA shield faeces collection tube (Corp, Irvine, CA) (full name, date of birth and collection date will be pre-labelled), (2) instructions on how to use the stool collection kit, (3) a pair of disposable latex gloves, a ziplock bag and a flushable faeces catcher, (4) a Bristol stool chart51 and (5) a Rome IV criteria checklist.52 Based on the Rome IV criteria checklist, ensuring that the participant does not have irritable bowel syndrome is important. The DNA/RNA shield faeces collection tubes used in this study are designed to preserve nuclei acids in the stool sample and maintain stability at room temperature. Once the participants collect the stool sample, they will contact the researcher immediately for transportation arrangements to the lab. The stool sample can be stored at room temperature before shipment. Once delivered to the laboratory, the stool sample tubes will be frozen at −80°C until processing and analysis for gut microbiota. Participants who are withdrawn from the study will be replaced. The enrolment period is expected to last approximately 8 months.
Gut microbiota analysis
The microbial DNA of the stool samples will be extracted using the ZymoBIOMICS DNA Miniprep Kit (Corp, Irvine, CA), according to the manufacturer’s instructions. For 16S amplicon sequencing, library preparation will be conducted using an Illumina platform by a commercial provider (to be determined). The standard protocols will be employed to sequence 16S rRNA targeting V3-V4 region to characterise the gut microbiota composition. All raw sequencing data will undergo multiple quality control steps to eliminate adaptors and primer sequences and be trimmed to maintain sequence qualities above a phred score of 30. A longitudinal analysis of gut microbiota data, including the alpha and beta diversity changes from baseline to 12 weeks in two YBG interventions and the placebo groups, will be carried out on Qiime2 (V.2023.9 or later)53 using default settings. Permutational multivariate analysis of variance (PERMANOVA) will be applied to identify the significant differences in gut microbial communities between intervention and placebo groups. For microbial community metabolic function examination, the top 500 taxa representative sequences from the two YBG interventions and placebo groups will be inferred from the Kyoto Encyclopaedia of Genes and Genomes (KEGG) using PICRUSt2 pipeline.54 To improve the accuracy and reliability of the KEGG pathways, a web-based tool, microbiome analyst55 designed for the comprehensive statistical, visual and meta-analysis of microbiota data will be used to remove extremely low-abundance and low-variance KEEG orthologs and Clusters of Orthologous Groups from each sample, respectively. This interpretation offers valuable insights into the overall structure and dynamics of the gut microbiota.
Compliance and adverse effects monitoring
Compliance checking
The participants from all three groups, two YBG interventions and a placebo group will receive 2 g×90 sachets at baseline and week 6 for a supply of 12 weeks. Compliance will be assessed by performing a sachet count and checking the daily dose diary on week 6. Considering the evidence of the previous RCT studies, participants who consumed <75% of the recommended intake of the study supplement doses will be excluded for non-compliance. The researcher will remind the participants to take the sachets regularly as per the previous instructions through daily phone calls and short message service/WhatsApp messages.
Prohibited concomitant medication and interventions
The following concomitant medications and interventions are not permitted while the participant is on trial:
Steroid medication (cortisone, hydrocortisone and prednisone)
Immune-modifying medication (antibiotics)
Dietary supplements (probiotics, prebiotics, vitamin A, vitamin B complex, vitamin C, vitamin D, selenium, zinc supplements)
Herbal products which can influence immune systems (Echinacea, black seed oil)
Influenza vaccination for the past 1 year and Pneumococcal vaccine for the past 5 years
At each visit, new or changes in concomitant medications will be documented.
Adverse events
The tolerability of participants towards the study supplement will be assessed by reviewing vital signs and adverse event (AE) reports. The frequency and intensity of AEs and serious AEs will be recorded in detail, based on the participant’s feedback during each visit. To obtain comparable documentation on AEs, the investigator will inquire with participants using standardised, open-ended questions during each visit based on the AE form (online supplemental appendix 4). Even though not anticipated, the serious adverse events will be immediately reported to the principal investigator and manufacturer of the study supplement for safety reasons.
Follow-up visits
All study participants will be followed up for the duration of 12 weeks which includes follow-up via call or WhatsApp during week 4 and direct follow-up visits at week 6 and week 12, as shown in table 3.
Summary of activities and parameters involved according to study period data collection
Statistical analysis
Statistical Package for the Social Sciences programme, V.23.0, will be used to analyse the data. The data will be presented as mean±SD for parametric data and median (range) for non-parametric data. The normality of data distribution will be tested using the Shapiro-Wilk test. A p value of <0.05 will be used to represent statistical significance. Descriptive analysis including frequencies, percentage, mean and SD will be used to analyse sociodemographic data and blood pressure.
To determine the effects of YBG on outcomes, a mixed-design ANOVA will be used to compare the intervention group and the control group to determine the interaction effect of intervention and time. Subgroup analysis according to gender, age group and stress level will also be conducted.
Recruitment and public involvement
To maintain the recruitment rate, recruitment progress will be assessed on monthly basis to troubleshoot the issues that contribute to slow data collection if there are any. Community-based events and general health screenings related awareness talks will be conducted to attract potential study subjects. The results and findings of the study will be shared with enrolled participants by debriefing presentation.
Ethics, amendments to the protocol and dissemination
The study’s research protocol including the Informed Consent Form and Participant Information Sheet was reviewed and approved by the Research Ethics Committee of the National University of Malaysia (Universiti Kebangsaan Malaysia) (JEP-2023–211). The study protocol has also been registered at the International Standard Randomised Controlled Trial Number (ISRCTN) Registry (ISRCTN48336189). Verbal and written information regarding informed consent will be shared with potential participants before enrollment. Any protocol modifications will be submitted to the ethics committee and study registry for review and approval. Any necessary changes must be signed by the principal investigator and the researchers. If the modifications are significant and are likely to have an influence on the participants’ safety, or if they adjust the interpretation of scientific materials used to support the trial’s conduct, the changes will be notified to the relevant ethics committee. The results of this RCT study will be disseminated to the public through open-access journals and conference presentations.
Trial status
The recruitment of the participants is going to begin in February 2024. Estimating to end the data collection by November 2024.
Discussion
This study aimed to determine the efficacy of low dosage YBG 1,3/1,6 on URTI symptoms, fatigue level, immune response and gut health among moderate-stress adults. Most RCTs indicate an intensified effect of oral fungal or YBG supplementation on the immune defence system targeted among healthy adults. However, this study is one of the very few studies that includes adults with high susceptibility to respiratory infection. Nevertheless, this study is warranted as there is a gap in human research regarding YBG and its impact on the gut microbiota. Despite its potential significance, no human studies have comprehensively investigated this aspect, highlighting the need for this study to bridge the existing knowledge gap.
Notably, none of the previous studies reported adverse effects causally related to YBG supplementation. The treatment was well tolerated in all the different populations, regardless of variation in age, sex and health status. This is an important observation that favours the use of YBG supplementation for many purposes, as it is supported by a significant number of studies with great variability in population. This study focused on South East Asia’s multiethnic population, specifically in Malaysia. Considering prior research in Western, European and diverse Asian countries, including China and Japan, it is essential to conduct this study within the Southeast Asian population. This will allow us to assess YBG’s effectiveness on our specific population.
Based on the evidence and human clinical study that will be conducted, we expect that the findings of this study will provide a positive effect on the value of immunonutrition supplementation using YBG 1,3/1,6 at a lower dosage on URTI, fatigue, immune response health outcome and be able to generate new insights about the mechanism of action of YBG 1,3/1,6 in the microbiota immune axis.
Limitations of the study
This 12-week trial may have challenges with participant compliance and incomplete data, which could reduce statistical power and introduce bias. Variability in supplement adherence and participant dropouts may jeopardise the validity of the findings; however, using reminders and regular checks via WhatsApp may improve adherence and reduce dropout rates.
Furthermore, hesitancy in providing stool samples can significantly reduce recruitment rates and sample size, ultimately limiting the study’s validity of the results. Because stool samples are crucial for analysing gut microbiota, participant discomfort presents a potential obstacle. Therefore, clearly explaining the significance of the stool sample for the study can motivate participants by emphasising how their contribution supports research and may lead to potential health benefits.
Implication and future implementation
The study on YBG holds significant implications and potential for future implementation in various aspects of health and research. This study will enable us to understand the immune-boosting properties of YBG, which can lead to the development of immune-boosting supplements or dietary interventions to enhance overall immune health and combat infections. Besides, given its potential to bolster the immune response, YBG could offer a supportive approach to managing respiratory infections, especially in vulnerable populations. Also, the study could pave the way for incorporating YBG into health supplements and functional foods, promoting its widespread use for its immune-enhancing benefits. Research may unveil the preventive potential of YBG in various diseases, allowing for its inclusion in preventive healthcare strategies. Future research should involve well-designed clinical trials to establish the efficacy and safety of YBG at various dosages. This will provide concrete evidence for its potential applications. Governments and healthcare organisations could consider incorporating YBG as part of public health initiatives to strengthen immune responses, particularly during disease outbreaks. Last but not least, the food industry can be encouraged to integrate YBG into various foods, offering a convenient way for individuals to enhance their immune systems through their daily diet. In summary, the study on YBG has promising implications for immune health enhancement and disease management.
Ethics statements
Patient consent for publication
Acknowledgments
The authors thank the staff of the Centre for Healthy Aging and Wellness (H-Care), Faculty of Health Sciences, Universiti Kebangsaan Malaysia and others for assistance in preparing the study protocol.
References
Footnotes
Contributors All authors made substantial contributions in the process of critical revision of the article for optimum intellectual content. NNMH drafted the article under supervision of SS. NNMH, SS, MI, MZAK, NI, SGHT, MFAH and KR were involved in designing the study protocol and conceptualisation. NNMH conducting the participants recruitment under supervision of SS, MI, SGHT, MFAH and KR. MFAH are the internal medicine physician who will be actively involved in monitoring any adverse effects that may occur during the research. For data analysis using Statistical Package for the Social Sciences software, SS, MI, NI, SGHT and KR will guide NNMH with their knowledge and experience in performing statistical analysis. SS is responsible for the overall content as the guarantor. All authors have read, edited and approved the final draft of this article.
Funding This work was supported by an educational grant from Mead Johnson Nutrition Sdn. Bhd. Kuala Lumpur, Malaysia. Grant number: NN-2022-027.
Disclaimer This research is supported by an educational grant, with the sponsor participating solely in general discussions. The sponsor's involvement is limited to financial support for the research.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.