Article Text
Abstract
Background Cervical cancer (CC) is preventable through regular screening and vaccination against human papillomavirus (HPV). However, CC remains a significant public health issue in low-income and middle-income countries (LMICs) like Vietnam, where financial constraints hinder the widespread implementation of HPV vaccination and screening programmes. Currently, Vietnam lacks both a national CC screening intervention and an HPV vaccination programme for women and girls. To date, cost-effectiveness studies evaluating CC screening methods in Vietnam remain limited.
Objectives To evaluate the cost-effectiveness of two CC screening strategies for Vietnamese women aged 25–55 years: (1) cotesting combining cytology and HPV testing conducted three times at 5 year intervals (intervention) and (2) cytology-based screening conducted five times at 2 year intervals (comparator). The objective is to provide evidence to inform policy and clinical practice in Vietnam.
Design Cost-effectiveness analysis using a Markov model with 1 year cycles to simulate the natural progression of CC.
Setting The Vietnamese healthcare system, modelled from the provider’s perspective, with parameters adapted to the local context through expert consultations.
Participants A simulated cohort of Vietnamese women aged 25–55 years.
Interventions The intervention involved cotesting (cytology and HPV testing) three times at 5 year intervals. The comparator was cytology-based screening conducted five times at 2 year intervals.
Primary and secondary outcome measures The primary outcome measure was quality-adjusted life years (QALYs). Costs and cost-effectiveness ratios were assessed using Vietnam’s gross domestic product (GDP) per capita as the cost-effectiveness threshold (1–3 times GDP per capita). Sensitivity analyses (one-way deterministic and probabilistic) were conducted to account for uncertainties.
Results The cotesting strategy was less effective and more costly than cytology-based screening across all age groups. Cotesting resulted in higher costs and fewer QALYs than the comparator. Probabilistic sensitivity analyses confirmed that cotesting was not cost-effective under current conditions in Vietnam.
Conclusions Cytology-based screening conducted five times at 2 year intervals is a more cost-effective option for CC screening in Vietnamese women aged 25–55 years. The cotesting strategy cannot be recommended due to its higher cost and lower effectiveness.
- Health economics
- PUBLIC HEALTH
- ONCOLOGY
- Mass Screening
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. The authors confirm that the data supporting the findings of this study are available within the article (and/or) its supplementary materials.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTH AND LIMITATIONS OF THIS STUDY
The screening age interval was informed by WHO recommendations, human papillomavirus (HPV) prevalence data in Vietnamese women, and a systematic review, but the lack of robust local evidence may limit its applicability.
The impact of HPV vaccination on the target population was excluded from the analysis because of insufficient available data.
The quality-adjusted life years used in this study were calculated using the standard gambling method, which may introduce variability in comparison with other health utility measurement techniques.
Transition probabilities were adapted from the ATHENA (The Addressing the Need for Advanced HPV Diagnostics study) trial, reflecting a population with low HPV vaccine coverage, aligning with the Vietnamese context but potentially limiting generalisability.
Introduction
According to global statistic on cancer, cervical cancer (CC) poses a considerable disease burden for low-income and middle-income countries (LMICs), but it can be effectively controlled through widespread coverage of HPV vaccine and cervical screening programmes.1 2 In Vietnam, CC ranks among the most prevalent cancers affecting women.3 4
From 2016, Vietnam Ministry of Health (MOH) approved a national action plan for the prevention and control of CC,covering the period from 2016 to 2025. However, funding for the screening programme has not been incorporated into the national health insurance, relies instead on local budgets and official development aid sources. This unstable budget allocation presents a significant challenge to achieving the objectives outlined in the action plan. According to the guidelines for control and prevention of CC from MOH, screening methods are recommended for women aged 21–65 years and include (1) cervical cytology, (2) cervical observation with axis acetic (VIA, visual inspection with acetic acid) or examination of the cervix with Lugol’s solution (VILI, visual inspection with Lugol’s Iodine) and (3) test to detect human papillomavirus (HPV) types. These tests can be utilised either independently or in combination, with intervals ranging from 2 to 5 years. Specifically, cotesting, which combines HPV testing with cervical cytology, allows for an extended screening interval of up to 5 years, thereby reducing the overall number of screenings in a woman’s lifetime. Among the available screening methods, colposcopy is considered the least effective for early diagnosis of CC and is primary recommended in low-income countries.5 Cytology and HPV testing are widely used and recommended as primary methods in numerous national programmes. While HPV testing is characterised by lower specificity, the sensitivity of cytology is highly contingent on quality control and assurance measures. A review by Herbert et al indicated that the limitations of HPV testing may be offset by cytology and vice versa.6 Thus, the combination of these tests (cotesting) represents a promising approach in populations with low HPV vaccination prevalence.6 Given that Vietnam is a LMIC with low HPV vaccination coverage, there is an imperative to identify a primary cervical screening method that is both effective and cost-efficient. Recent studies have demonstrated that cotesting is more cost-effective than cervical cytology alone, owing to reduced screening cycles and improved sensitivity and specificity.6–13
Following Vietnam MOH guidelines, the cotesting method is recommended for women aged 30 years old and older.14 However, HPV infection can occur at younger ages; the average age of first sexual initiation among Vietnamese youth was reported to be 18.7 years in 2018.15 Women infected with HPV may develop CC within 10–20 years depending on their immune response, with a more rapid progression likely in individual with compromised immune systems. Consequently, implementing a CC screening programme for younger women is essential for preventing disease progression.16 Current guidelines in the USA, Europe and Australia advocate for the initiation of CC screening at the age 25.17–20 In high-income countries (HICs), women aged 21–65 years undergo routine CC screening at interval of 3–5 years, depending on the primary screening method.17–20 However, this screening strategy poses a challenge for LMIC due to financial constraints and scarcity of infrastructure.21–24 In LMIC, WHO suggested that women aged 35–54 years with adequate screening history and no abnormal results may safely discontinue screening, given their low risk for HPV infection.25 An adequate screening history is defined as three consecutive negative cytology results or two consecutive negative cotesting results within the past 10 years, with the most recent test performed within the last 5 years.18 Accordingly, this study proposes an alternative screening strategy whereby women aged 25–55 years undergo cotesting three times consecutively at 5 year interval. For example, a 25-year-old woman will be screened CC at the age of 25, 30 and 35. Should this approach be found cost-effective, further research will be necessary to explore whether adjustments to the screening age interval might yield even greater cost-effectiveness.
To date, only two studies have investigated on the cost-effectiveness of cervical cytology in Vietnam: one conducted by Suba et al in 2001 and another by Kim et al in 2008. Suba et al concluded that cervical cytology was the optimal screening method at that time due to a lack of resources for other screening methods.26 Kim et al study mainly focused on the cost-effectiveness of HPV vaccination in combination with three times CC screening in a whole woman life or once in every 5 years in Vietnam.27 However, research assessing the cost-effectiveness of cervical screening methods in Vietnam remains limited, particularly concerning newer approaches such as cotesting. Therefore, this study aims to investigate the cost-effectiveness of CC screening three times consecutively at 5 year interval using the cotesting method compared with five times consecutively at 2 year interval using the cytology method for Vietnamese women aged 25–55 years.
Methods
Study design
The study applied a cost-effectiveness analysis (CEA) method using Markov modelling to assess the efficiency of the cotesting method in CC screening for Vietnamese women from 25 to 55 years old. The analysis stimulated a cohort of 1000 women at the age of 25 and followed them through yearly cycle until they reached their upper limited screening age. For instance, the group aged 25–29 was modelled and analysed until they turned 55, as was the group aged 30–34, and so forth for the remaining age groups. We applied the provider perspective for the cost-effective analysis.
Input parameters for the Markov modelling were obtained through a comprehensive literature review, drawing from common CEA models that simulate the natural progression of HPV infection. These inputs included transition probabilities, efficacy of screening methods, cost for treatment and screening tests and quality-adjusted life years (QALYs) for various health states. Medical and financial experts in the Viet Nam National Cancer Hospital (K hospital) were consulted to validate and adjust the input parameters. Then, a Microsoft Excel template was used to perform the simulation and calculate an incremental cost-effectiveness ratio (ICER).
Due to the differing transition probabilities across age groups, we developed six distinct Markov models for cohorts of 1000 women in each age group (25–29, 30–34, 35–39, 40–44, 45–49 and 50–55) to resemble the target women population from 25 to 55 years old. These groups underwent CC screening either three times consecutive using the cotesting method or five times consecutive using the cytology method. For example, the 25–29 age group was screened at the ages 25, 30 and 35 with cotesting and at the ages 25, 27, 29, 31 and 33 with cytology. The Markov model applied the same transition probabilities based on the starting cohort age and simulated the incidence of CC by age 55. Consequently, older cohorts underwent fewer screening rounds. Specifically, women aged 50–55 were screened only once using co-testing and twice using cytology.
Patients and public involvement
No patients or members of the public were involved in the design, conduct, reporting or dissemination plans of this study.
Study population
The incidence of CC in Vietnam increased rapidly in women aged 30–34, peaking in the 55–59 age group before gradually decreasing in older populations.28 Since CC typically develops many years after HPV infection, and the average age of first sexual initiation in Vietnam was 18.7 years,15 women aged from 25 to 55 years were identified as the target group for this study. This group faces a higher risk of CC development due to lack of HPV vaccination coverage and the absence of national health insurance reimbursement for CC screening in Vietnam.
To conduct the evaluation, we constructed a hypothetical cohort of one million women aged from 25 to 55, representing those at high risk of CC. These women were assumed to follow the same natural history of disease, screening, diagnosis, and treatment pathways.
Intervention and comparator
The cotesting method has been shown to be more cost-effective than cytology or HPV testing alone in routine CC screening up to the upper screening age limit in HICs.29–31 However, the LMICs cannot implement a routine CC screening for all target women due to limited resources. WHO and previous studies have recommended at least three consecutive screenings as a feasible strategy for CC screening in LMICs.18 25 This study investigates the cost-effectiveness of the cotesting method in Vietnam compared with the cytology method, which has been proven cost-effective for over a decade.
The research uses the Markov model to compare two screening strategies for Vietnamese women aged 25–55:
Cotesting method: combined cytology and HPV test, performed three times with a 5 year interval.
Cytology method: performed five times with a 2 year interval.
For example:
The 25–29 group was screened at ages of 25, 30 and 35 using the cotesting method, and at ages of 25, 27, 29, 31 and 33 using the cytology method.
The 30–34 group was screened at ages of 30, 35 and 40 using the cotesting method, and at ages of 30, 32, 34, 36 and 37 using the cytology method.
Markov model
The model and time horizon were based on the natural epidemiology of CC progression, reviewed from medical literature and results from randomised control trials and retrospective cohort studies.13 31–39 Most reviewed studies used Markov model with 1 year cycles for analysis, which was also adopted for this study. Additionally, high-risk HPV (HPVhr) natural history models were reviewed, and clinical experts were consulted for adjustment.24 40–50 Since the proposed Markov model shared similar health states with other models, clinical experts did not suggest any changes.
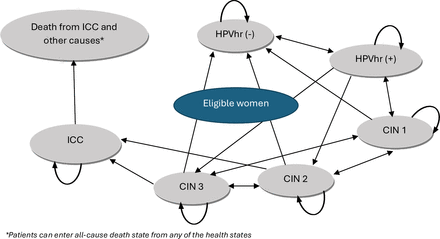
The Markov model with seven states was used to simulate the natural disease progression of HPV infection with 1 year cycles (figure 1). The Markov model was based on following assumptions:
A cohort of 1000 undiagnosed women, aged 25–55 and engaging in sexual activity, would be screened using both the cotesting and cytology methods without adding new cohorts.
None of the women were vaccinated against HPV.
None of them has experienced hysterectomy.
Full participation in screening programmes was assumed.
All detected cases of HPV positive, cervical intraepithelial neoplasia (CIN) 1, CIN 2, CIN 3 and CC would receive treatment.
Markov model of Felix et al (2016). CIN, cervical intraepithelial neoplasia; HPVhr (−), HPV high-risk negative; HPVhr (+), HPV high-risk positive; ICC invasive cervical cancer.
QALYs were used to measure health outcomes. The Markov model was developed with health states based on history of natural HPV infection which adopted from Felix et al (2016).13
Input parameters
The effectiveness of screening tests, defined by their sensitivity and specificity, is detailed in online supplemental table S1. These data were obtained from systematic reviews and meta-analyses.51 52 Screening costs were sourced from Circulars no 13/2019TT-BYT, which regulates medical service prices in Vietnam (https://thuvienphapluat.vn/), and treatment costs were drawn from Nguyen et al’s study on the medical costs of treating CC at central hospitals in Vietnam, analysed from a provider perspective (online supplemental table S2). Because treatment cost for CC varied according to severity level and treatment pathways for CIN 1, CIN 2 and CIN 3 were similar; hence, we used average cost of scenarios to generate the treatment cost for these states. The cost inputs were adjusted to 2022 values based on Consumer price index published by Viet Nam General Statistics Office.53
Supplemental material
Supplemental material
Currently, there are no Vietnamese data on QALY weights for the different states in the Markov model. Similar cost-effectiveness analyses of CC screening in China, Taiwan and Thailand used utility weights from clinical trials conducted in countries such as the USA, Canada and the UK. In this study, we applied data from Warner et al,34 which derived QALYs for CIN 1, CIN 2, CIN 3 and CC states from population surveillance in Canada and the USA using the standard gamble method (online supplemental table S3). The QALY for CC state was averaged across treatment phases in accordance with the International Federation of Obstetrics and Gynaecology states to avoid over estimation utility for CC.
Supplemental material
For transition probabilities related to disease progression and regression (online supplemental table S4), we consulted data from the study ‘Screening for Cervical Cancer: A Decision Analysis for the U.S. Preventive Services Task Force’ and the study ‘The Clinical and Economic Benefits of Co-Testing vs Primary HPV Testing for Cervical Cancer Screening: A Modeling Analysis’.13 54 These studies used data from the ATHENA trial (The Addressing the Need for Advanced HPV Diagnostics study) conducted in a population with low HPV vaccination coverage (2%), making the data suitable for the Vietnam context.55
Supplemental material
Cost-effectiveness analysis and sensitivity analysis
After adjusting all costs for inflation to 2022 and applying a discount rate of 3%, an ICER was computed to evaluate efficiency of the cotesting method compared with the cytology method. Up to date, there are no specific ICER thresholds for CEA in Vietnam. Therefore, we followed the older recommendations of WHO, which suggested that if the ICER was lower than 1–3 gross domestic product (GDP) per capita, the intervention may be considered cost-effective.56–58 According to the GDP data of Vietnam published by the World Bank in 2022, it was US$2785.7/per capita.59 The exchange rate used for converting VND to USD was 23 060 VND per USD, based on Vietcombank’s rate.60 Thus, 1 GDP per capita equals 63.68 million VND (US$2786) and 3 GDP per capita equals 191.04 million VND (US$8357).
For sensitivity analysis, we conducted both probabilistic sensitivity analysis (PSA) and deterministic sensitivity analysis (DSA). PSA was performed using Monte Carlo simulations (1000 iterations) to present probabilities of cost-effectiveness (%) and the cost-effectiveness acceptancy curve (CEAC) corresponding to the Vietnam’s GDP thresholds, to illustrate the impact of uncertainty on the ICER. In the one-way DSA, all parameters, including the cost of the cotesting and cytology methods, treatment services and QALYs, were analysed. We identified 15 influential parameters and evaluated the impact of changes in the number of cytology screening rounds on the ICER. The results were presented using Tornado diagrams.
Results
Incremental cost-effectiveness ratio (ICER)
The results showed that cotesting was not cost-effective compared with cytology (table 1). The total screening costs and QALYs for each age group was summarised in table 1. In general, in five age groups (25–29, 35–39, 40–44, 45–49 and 50–55), cotesting were dominated by cytology, meaning that cotesting had higher cost and lower QALYs. In the 25–29 group, the total cost of the cotesting programme was highest with US$1266 with a total QALYs of 24.72. While the cytology programme cost less at US$1122 and provided higher QALYs at 26.34.
Cost, QALY weights and ICER between alternatives (3% discount)
For the 30–34 age group, the cost of cotesting was lower than cytology; however, switching from cytology to cotesting would result in a QALY loss. Cotesting could be a cost-effective alternative if the savings per QALY lost exceeded the threshold, but this was not the case here, as Vietnam’s GDP per capita was US$2786, and the saving cost was only US$3.
For both screening strategies, the total cost for other age groups decreased significantly. In the cotesting programme, the total cost decreased from US$516 to US$198, while the QALYs dropped from 18.59 to 6.07. Similarly, in the cytology programme, the total cost decreased from US$484 to US$146, with QALYs falling 20.54 to 7.26.
Despite higher cost, the cotesting method reduced the incidence of CC and CIN 1/2/3 cases more effectively than cytology. The cotesting prevented 887 CIN 1/2/3 cases and 32 CC cases, compared with 627 CIN 1/2/3 cases and 24 CC cases prevented by cytology, when compared with a no-screening scenario. But the number of false positive cases detected by the cotesting method was double that of the cytology method. The cost for older age groups decreased significantly because the model only stimulated the cohort until they reached 55 years old, resulting in fewer screening cycles for older women and, consequently, lower cost.
Sensitivity analysis
In the one-way DSA, 23 parameters from cost, transition probabilities and QALYs were analysed to find the most influential parameters. After evaluating the differences in ICER, 15 influential parameters were identified. In general, the top 15 influential factors varied among age groups. The Tornado diagrams revealed that the ICER was most sensitive to the transition probability from HPVhr (−) state to HPVhr (+) state and the prevalence of HPVhr cases in general female population across all age groups (figures 2–4). The cost and effectiveness of screening tests has a smaller impact on ICER changes in most age groups, except in the 50–55 age group, where the cost of cotesting ranked third, while the costs of the HPV and cytology tests ranked sixth and seventh, respectively.
One-way deterministic sensitivity analysis results in 25–29 and 30–34 age groups. CC, cervical cancer; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ICER, incremental cost-effectiveness ratioQALY, quality-adjusted life years.
One-way deterministic sensitivity analysis results in 35–39 and 40–44 age groups. CC, cervical cancer; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years.
One-way deterministic sensitivity analysis results in 45–49 and 50–55 age groups. CC, cervical cancer; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years.
The treatment cost of CC had a significant impact on the ICER, particularly in the 35–39 age group, where it ranked second, and it ranked third in the 25–29, 30–34 and 40–44 groups and fourth in the 45–49 group. However, in the 50–55 age group, this parameter had little influence on the ICER changes.
Cost effectiveness planes for 1000 Monte Carlo simulations and CEAC are shown from online supplemental figures S1–6. The ICER simulations fluctuated primarily in the north-west and south-west quadrants, indicating that the strategy of three times consecutive screening via cotesting was dominated by the comparator (cytology). The CEACs also confirmed the robustness of the PSA and DSA results, showing that the probability of the ICER remaining below Vietnam’s willingness-to-pay (WTP) threshold (1–3 times GDP per capita) was relatively low, at around 40%–50% across all age groups. The probability of cotesting achieving cost-effectiveness approached 0% as the WTP threshold increased beyond US$1200. In contrast, the cytology method demonstrated a higher probability (40%–60%) of achieving cost-effectiveness, with the CEAC for cytology approaching 100% at the WTP threshold. This suggests that cytology is more likely to be cost-effective, even when the WTP is lower than 1 GDP (US$2786).
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
To analyse the impact of the number of CC screening rounds on the ICER, an additional scenario was conducted to compare cost-effectiveness of three times consecutive cotesting and three times consecutive cytology for Vietnamese women 25–30 years old. In this scenario, the cotesting method (cost: US$1202 and QALYs: 24.18) was superior to the cytology method (cost: US$955 and QALYs: 22.55) with an ICER of 152 USD per QALY gained which is lower than 1 GDP per capita (US$2786). Under uncertainty conditions, 1000 Monte Carlo simulations for this scenario were concentrated in the north-east quadrant, indicating that the number of screening rounds significantly impacts the ICER.
Discussion
Main findings
Based on our analysis in the results part, CC screening by three times consecutive cotesting was not cost-effective compared with CC screening by five times cytology. While the cotesting strategy prevented more cases of CIN 1/2/3 and CC than the cytology method, the cost was significantly higher. Additionally, the large number of false positives detected by cotesting raised concerns about its potential impact on patients’ mental health due to overtreatment, and it posed a financial burden on the public health budget. The one-way DSA indicated that the ICER was most sensitive to the transition probability from HPVhr (-) to HPVhr (+) and the prevalence of HPVhr in the general population. Conversely, the cost and effectiveness of screening tests had minimal impacts. The PSA and CEAC further confirmed these findings. At Vietnam’s WTP threshold, the probability of cost-effectiveness for cotesting was close to 0, whereas the cytology method had a 100% probability of being cost-effective at around US$1200, well below 1 GDP per capita (US$2786).
Our study proved that the cotesting was dominated by the cytology and this result contrasted with findings from studies of Ian Cromwell et al (2021), Adam Keane et al (2020), Anastasios Skroumpelos et al (2019), Taejong Song et al (2018), Jie-Bin Lew et al (2017) and Thomas Wright et al (2016). Those studies indicated that cotesting was cost-effective in preventing CC incidence and deaths compared with cytology. However, when the comparator was the HPV test, the difference in results was not as significant. Among all screening strategies, cotesting was associated with the lowest CC incidence and high performance in detecting early-stage CC. Although cotesting gained more QALYs, this difference was not significant, and it came with the highest cost per screened woman, a critical consideration for policymakers.29–32 36 39
Several factors may explain the differing outcomes. First, our study used a 2 year screening interval for cytology, whereas most other studies used a 3 year interval. A shorter screening interval could lead to fewer missed diagnoses in the simulated cohorts, resulting in higher QALY weights. Second, the frequency of CC screening also differed: previous studies considered screening throughout a woman’s lifetime until she reached the upper age limit, whereas our model focused only on three or five consecutive screenings. Consequently, women over the age of 49 received fewer screenings in our model, particularly those in the cotesting group, even though the incidence of CC in Vietnam peaks in women over 45.28 This reduction in screening for older women resulted in smaller QALY gains for the cotesting group.
In the DSA, we found out that the ICER was the most sensitive to the transition probability from HPVhr (−) state to HPVhr (+) state, the prevalence of HPVhr in general women population and the number of CC screening rounds. Although we cannot influence the transition probability, the other factors could be addressed through expanded HPV vaccination programmes and implemented CC screening strategies. Vietnam could increase HPV vaccination coverage by negotiating lower vaccine prices, potentially at US$4.55 per dose (65–67). These factors influence the ICER range, which still falls below Vietnam’s current cost-effectiveness thresholds (1–3 GDP per capita). However, increasing the number of routine cytology screenings could reverse the cost-effectiveness results, making cytology more favourable than cotesting. Future research should explore the optimal number of cytology screenings needed to achieve greater cost-effectiveness compared with cotesting.
Our study demonstrated that cotesting was dominated by cytology across all age groups, resulting in lower QALY gains. If we reversed the intervention and comparator in the study, the savings would range from US$16 to US$64 per QALY gained. However, in the second scenario (three rounds of cotesting vs three rounds of cytology), the ICER was US$152 per QALY gained, meaning that women would need to pay US$152 to gain 1 QALY, whereas they could save money with the alternative approach. The findings from both scenarios show that while cotesting can reduce CC incidence, it comes at a much higher cost. Another significant drawback of cotesting is the increase in false positives, which adds to the financial burden due to the need for triage and follow-up tests. These results align with previous studies that highlight the medical benefits of cotesting, although at a substantially higher cost.29–32 36 39
Strength and limitations
The study noted some limitations. First, this research proposed an age interval from 25 to 55 years for CC screening, while national guidelines from other countries targeted women from 21 to 65 or even up to 79 years. Therefore, future research should investigate the appropriate age interval for CC screening in Vietnam. Second, the research did not consider effects from HPV vaccination on the transition probabilities of the Markov model. Although the prevalence of HPV vaccinated women in the target group in Vietnam is unknown, it might decrease sensitivity and positive predicted value of the cytology method. To adjust the input parameters, we used data from studies about efficacy and effectiveness of cytology-based screening method in LMICs.
Third, for the 21 transition probabilities that were used in the Markov model, only one assumption about the lower and upper ranges was made. This assumption was based on the study ‘Effectiveness and cost-effectiveness of eliminating cervical cancer through a tailored optimal pathway: a modelling study’ in China which applies ±25% for the range of transition probabilities.61 We also validated this percentage point and the secondary data with four Vietnamese clinical experts to ensure the best available evidence to the researcher’s knowledge. However, this limitation suggests the need for more localised data, specifically on transition probabilities.
Another limitation in our study was the QALY weights which were sourced from the study from a large population in Canada and USA. The weights were elicited by the standard gamble method,34 which is a valid approach but not the most commonly used in recent studies. Although we found no substantial differences between QALY weights from the EQ-5D method and the standard gamble method.13 34 62–64 Hence, we decided to use the data on QALY weights from Warner et al (2015). They still contributed to the limitations of our study and future studies should focus on deriving QALY weights more specific to the Vietnamese population.
To manage uncertainties, we used PSA. However, the original study about the transition probabilities of the Markov model did not publish the SD, 95% CI, range or full parameter distributions. As a results, we estimated these values based on the transition probabilities and the data from the studies ‘A dynamic Bayesian Markov model for health economic evaluations of interventions in infectious disease’ to generate β distribution for the PSA.13 65 Applying the same distribution for all age groups in the PSA might limit the precision of our results. There is a need to conduct systematic reviews and meta-analysis about the transition probabilities of CC development in the general population to address this in future studies.
Conclusion
The study found that three consecutive CC screenings using cotesting at 5 year intervals were less cost-effective and less beneficial than five screenings using cytology at 2 year intervals. Although the cotesting method was cost-effective in one scenario under DSA, it required women to pay US$152 per QALY gained (ICER=US$152). For the base-case analysis, using cytology as the intervention could lead to savings ranging from US$16 to 64 per QALY gained, which would be appealing for both healthcare decision-makers and users. Additionally, cotesting resulted in a significant increase in referrals and unnecessary treatment of healthy women, posing a financial burden on Vietnam’s healthcare system and creating potential mental health concerns for patients.
Given the reasonable clinical benefits and cost savings per QALY gained, the strategy of five consecutive cytology screenings for women aged 25–55 years can be recommended for CC screening in Vietnam.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. The authors confirm that the data supporting the findings of this study are available within the article (and/or) its supplementary materials.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Ethical Committee of Hanoi University of Public health (no 154/2022/YTCC-HD3 on 20th May 2022). Participants gave informed consent to participate in the study before taking part.
References
Footnotes
Contributors HTB developed research proposal, reviewed literature and field data for model inputs; calibrated the model; performed model simulations and data analyses; drafted and revised the manuscripts; was responsible for the overall content as guarantor. VNHP provided technical advice related to Markov model, input data, model simulations and data analysis; reviewed and commented for the manuscripts. THV provided technical advice related to cervical cancer epidemiology in Vietnam, local information to make model assumptions and input data for the Markov model.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.