Article Text
Abstract
Introduction Compared with the operating room, tracheal intubations in the intensive care unit (ICU) are associated with worsened glottic view, decreased first-time success rate and increase in the technical difficulty of intubation and incidence of complications. Videolaryngoscopes (VLs) have been proposed to improve airway management, and while recent studies have confirmed that VLs improve intubation conditions in this patient population, there remains a lack of clarity regarding the selection between a standard Macintosh blade or a hyperangulated one, to determine which yields the best outcomes. The purpose of this study was to compare successful intubation on the first attempt with the Macintosh VL versus the hyperangulated VL during tracheal intubation in ICU patients. We hypothesise that tracheal intubation using the hyperangulated VL will improve the frequency of successful intubation on the first attempt.
Methods and analysis The INtubation VIdeolaryngoscopy BLADE-ICU trial is a prospective, multicentre, open-label, interventional, randomised, controlled superiority study conducted in 29 ICUs in Spain. Patients will be randomly assigned in a 1:1 ratio to undergo intubation using a Macintosh VL (control group) or a hyperangulated VL (experimental group) for the first intubation attempt. The primary outcome is successful intubation on the first attempt. The secondary outcomes include the time to intubation, attempts for successful intubation, laryngoscopic vision assessed with the modified Cormack-Lehane scale, the need for adjuvant airway devices for intubation, difficulty assessed by the anaesthesiologist and complications during tracheal intubation. Enrolment began on 1 May 2024 and is expected to be completed in 2025.
Ethics and dissemination The study protocol was approved on 29 February 2024, by the Ethics Committee of Galicia (CEImG, code No. 2024-031).
The results will be submitted for publication in a peer-reviewed journal.
Trial registration number NCT06322719.
- Adult intensive & critical care
- Adult anaesthesia
- Clinical Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The plan is to include at least 1036 patients in this multicentre, prospective, open-label, interventional, randomised study.
The trial aims to compare two different videolaryngoscopes (Macintosh vs hyperangulated) for tracheal intubation in intensive care unit.
Conduct in 29 different hospitals among anaesthesiologists with diverse prior experience with tracheal intubation will increase the external validity of results.
The nature of the study does not allow blinding.
Introduction
Background and rationale
Compared with the operating room, tracheal intubations in the intensive care unit (ICU) are associated with worsened glottic view, decreased first-time success rate and increase in the technical difficulty of intubation and incidence of complications.1–3 Videolaryngoscopes have been proposed to improve airway management, and the use of these devices is recommended as first-line or after a first-attempt failure using direct laryngoscopy in ICU airway management algorithms.4
Although until relatively a few years ago there were doubts about whether videolaryngoscopes had advantages over direct laryngoscopy for endotracheal intubation in critically ill patients, two recent studies (DEVICE trial,5 INTUBE subanalysis6 7), a Cochrane review8 and a meta-analysis9 have confirmed that these devices improve intubation conditions in this patient population. The DEVICE trial5 was a multicentre, randomised study conducted in the USA that compared videolaryngoscopy with direct laryngoscopy for tracheal intubation in 1417 critically ill patients. The use of a videolaryngoscope resulted in a higher incidence of successful intubation on the first attempt compared with direct laryngoscopy (85% vs 71%). The subanalysis of the INTUBE study6 was a prospective, cohort study conducted internationally with 2916 critically ill patients intubated in 29 different countries. A videolaryngoscope was used for endotracheal intubation in 500 patients (17%) while direct laryngoscopy was used in 2416 (83%). The use of videolaryngoscopy was associated with a higher incidence of successful intubation on the first attempt (84% vs 79%). In the Cochrane review,8 the use of videolaryngoscopy was associated with improved glottic visualisation and fewer failed intubation attempts and complications such as hypoxaemia when compared with direct laryngoscopy. Araújo et al9 performed a meta-analysis, including 14 randomised controlled trials and 3981 patients comparing videolaryngoscopy versus direct laryngoscopy in critically ill patients, and they demonstrated that videolaryngoscopy is a more effective and safer strategy compared with direct laryngoscopy for increasing successful intubations on the first attempt, improving glottic visualisation and reducing oesophageal intubations in critically ill patients. After these results, they recommend the routine use of videolaryngoscopy in critically ill patients.
While we are beginning to accumulate evidence supporting the use of videolaryngoscopes as the preferred devices for intubating critically ill patients in the ICU, there is still a lack of clarity regarding the choice between a standard Macintosh blade or a hyperangulated one,10 to determine which leads to the best outcomes. In the DEVICE trial,5 a standard Macintosh blade was used in 85% of patients intubated with a videolaryngoscope while a hyperangulated blade was used in 15%. In the INTUBE subanalysis study,6 a hyperangulated blade was used in 25% of patients intubated with a videolaryngoscope, and the standard Macintosh blade was used in 75%. These studies do not describe the reasons for choosing one blade over the other.
The purpose of this prospective multicentre randomised study is to compare successful intubation on the first attempt with the Macintosh videolaryngoscope versus the hyperangulated videolaryngoscope during tracheal intubation in ICU patients. We hypothesise that tracheal intubation using the hyperangulated videolaryngoscope will improve the frequency of successful intubation on the first attempt in critically ill patients requiring intubation in the ICU.
Study aims and objectives
Primary objective
The main objective of this study is to determine whether hyperangulated videolaryngoscopy improves the frequency of successful first-pass tracheal intubation compared with Macintosh videolaryngoscopy in critically ill patients requiring intubation in the ICU.
Secondary objectives
The secondary objective is to compare the time to intubation, attempts for successful intubation, laryngoscopic vision with the modified Cormack-Lehane scale, the need for adjuvant airway devices for intubation, difficulty assessed by the anaesthesiologist and complications during tracheal intubation, between the hyperangulated and the Macintosh videolaryngoscopes.
Study design
The INtubation VIdeolaryngoscopy BLADE-ICU (INVIBLADE-ICU) study is a prospective, multicentre, open-label, interventional, randomised, controlled superiority study.
Methods: participants, interventions and outcomes
Study settings
The INVIBLADE-ICU study will take place in 29 ICUs, in Spain.
Eligibility criteria
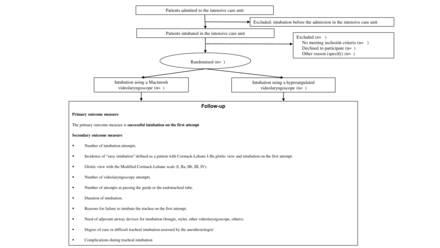
Inclusion criteria
Adult (age ≥18 years).
Patients were admitted to the ICU and required mechanical ventilation through an endotracheal tube.
Planned procedure is tracheal intubation using a videolaryngoscope.
Exclusion criteria
Patients who are intubated in a place other than the ICU (surgical area, emergency and hospitalisation floor) are not included.
Refusal of the patient or next of kin to participate in the study.
Pregnancy or lactation.
Need for tracheal intubation with a device other than the videolaryngoscope (fiberoptic bronchoscope, direct laryngoscopy, tracheostomy, etc.).
Emergent tracheal intubation that does not allow for the randomisation of the procedure (eg, because of cardiac arrest).
Assignment and intervention
Allocation
Patients eligible for inclusion will be randomly assigned in a 1:1 ratio to undergo intubation using a Macintosh videolaryngoscope or using a hyperangulated videolaryngoscope for the first attempt in permuted blocks of 2, 4 or 6, stratified and balanced by centre. The assignment to each type of videolaryngoscope will be generated using a computerised randomisation sequence, placed in sequential numbers in opaque envelopes and distributed to the participating hospitals.
Blinding
Blinding to the type of videolaryngoscope is only possible for the patient. The anaesthesiologist will be informed of the type of videolaryngoscope (hyperangulated vs Macintosh) prior to induction of anaesthesia.
Interventions
Before beginning enrolment, operators at each hospital received previously theorical introduction and hands-on training in the use of hyperangulated and Macintosh videolaryngoscopy, and information about the protocol of the study.
The experimental group involves tracheal intubation using a hyperangulated videolaryngoscope on the first laryngoscopy attempt, including devices such as the GlideScope, Storz C-MAC D-Blade and McGrath X blade, or any other videolaryngoscope with the same blade geometry. The control group involves tracheal intubation using a standard Macintosh videolaryngoscope on the first laryngoscope attempt, including devices such as the Storz C-MAC, McGrath MAC, GlideScope MAC or any other videolaryngoscope with the Macintosh blade geometry. In both groups, the trial recommends that the operator performing the first intubation attempt looking the video screen) (‘indirect laryngoscopy’). The trial protocol does not dictate the brand of videolaryngoscope that will be used, but the device used in each group will be recorded. All other aspects of the procedure will be at the discretion of anaesthesiologist, including sedative and neuromuscular drugs, preoxygenation, endotracheal tube size and the use of bougie or a stylet. The trial recommends using a stylet inside the tube or a bougie to facilitate intubation with the Macintosh videolaryngoscope. With the hyperangulated videolaryngoscope, the trial recommends a style inside the tube with an angulation of at least 30°–40° at the tip, a flexible-tipped bougie or another similar bougie, considering the hyperangulation of the blade. If the first attempt is unsuccessful, the anaesthesiologist may use any method of intubation on subsequent intubation attempts, including the use of a hyperangulated videolaryngoscope in the Macintosh videolaryngoscope group or use of a Macintosh videolaryngoscope in the hyperangulated videolaryngoscope group. The type of videolaryngoscope used during the first and final laryngoscopy attempts will be collected and reported.
Outcome measures
Primary outcome measure
The primary outcome measure is successful intubation on the first attempt defined as the placement of an endotracheal tube in the trachea following a single insertion of a laryngoscope blade into the mouth and either a single insertion of an endotracheal tube into the mouth or a single insertion of a bougie into the mouth followed by a single insertion of an endotracheal tube into the mouth.5
Secondary outcomes measure
Number of intubation attempts.
Incidence of ‘easy intubation’ is defined as a patient with Cormack-Lehane I–IIa glottic view and intubation on the first attempt.
Glottic view with the Modified Cormack-Lehane scale (I, IIa, IIb, III, IV).
Number of videolaryngoscopy attempts.
Number of attempts at passing the guide or the endotracheal tube.
Duration of intubation is defined as the time from insertion of the videolaryngoscope blade into the mouth until the endotracheal tube passes through the vocal cords.
Reasons for failure to intubate the trachea on the first attempt (inadequate view of the larynx, inability to intubate the trachea with an endotracheal tube, inability to cannulate the trachea with a bougie, attempt aborted due to a change in patient condition (eg, worsened hypoxaemia, hypotension, bradycardia, vomiting, bleeding), technical failure of the laryngoscope (eg, battery, light source, camera, screen) and others.
Need of adjuvant airway devices for intubation (bougie, stylet, other videolaryngoscope and others).
Degree of ease or difficult tracheal intubation assessed by the anaesthesiologist (without difficulty, mild, moderate and severe difficulty).
Complications during tracheal intubation and within 15 min after successful intubation (eg, desaturation <90%, desaturation <80%, systolic blood pressure <80 mm Hg, systolic blood pressure <65 mm Hg, oesophageal intubation, pulmonary aspiration, dental injuries, airway injuries, cardiac arrest and others).
Need to change the device for intubation (replaced by another videolaryngoscope, a different angled blade, requirement for a fiberoptic bronchoscope…).
Participant timeline
The participant timeline is described in table 1.
Participant timeline
Recruitment
Trial investigators identify consecutive patients admitted in 29 ICUs in Spain, who need to be intubated and meet the inclusion criteria. Patient enrolment began on 1 May 2024 and is expected to be completed in 2025.
Data collection, management and analysis
Data collection and management
All outcome measurements are recorded during and after the intubation procedure by an independent and trained observer using a specific paper-based case report form (CRF). Trained observers may include clinical personnel in the enrolling UCI unit, such as anaesthesiologists or nurses. Also, pseudonymised data are collected and managed using Research Electronic Data Capture (REDCap), a REDCap tool hosted at Sanitary Research Institute Foundation of Santiago (IDIS).12 13 REDCap is a secure, web-beased software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for data integration and interoperability with external source.
The database will be protected by encryption software and a single user password will be provided to each centre. Every intubated patient is coded with a specific patient number. Any protocol deviations are recorded on the eCRF, and a clinical research assistant ensure that all protocol deviations and adverse events are recorded in the database.
Access to data
Data safety, data quality and statistical analysis will be managed by two investigators (AE-G and OV), who are responsible for notifying any issues that may arise during the whole study. Data will be collected and stored according to good clinical practice guidelines and will be available to all participants’ study centres. Any issue occurring during the study will be reported to these two investigators. Data registered are described in tables 2–4.
Baseline, demographic and clinical characteristics of trial patients
Intubation characteristics
Primary and secondary outcomes by assigned treatment groups
Statistical methods
Description of the patient groups at baseline and time period for analysis
The baseline characteristics of all patients will be described. Categorical variables will be reported as absolute numbers (n) and percentages and quantitative variables as mean (±SD) or median (25th–75th percentile). Normality will be calculated with the Kolmogorov-Smirnov test with Lilliefors correction.
Primary analysis
The main analysis will be an unadjusted, intention-to-treat comparison of successful intubation on the first attempt comparing hyperangulated videolaryngoscopy with Macintosh videolaryngoscopy in critically ill patients requiring intubation in the ICU. A χ2 test will be used to compare the primary outcome between the two groups. Also, incidence rates, RR, risk differences and 95% CIs will be calculated.
Secondary analysis
Secondary outcomes
For secondary outcomes, we will conduct unadjusted, intention-to-treat analyses comparing patients randomised to the hyperangulated videolaryngoscopy group to patients randomised to the Macintosh videolaryngoscopy group. Continuous variables will be compared with the Wilcoxon‘s rank-sum test and categorical variables with a χ2 test or the Fisher’s exact test, according to the conditions of application. Between-group differences in continuous and categorical variables and the associated 95% CIs will be presented.
Per-protocol analysis
We will perform a per-protocol analysis comparing patients in the hyperangulated videolaryngoscopy group to patients randomised to the Macintosh videolaryngoscopy group (regardless of group assignment).
Effect modification (subgroup analyses)
We will investigate if predetermined baseline variables influence the effect of the study group on the primary outcome. To assess this, we will use a logistic regression model focused on the primary outcome of first-attempt success. The independent variables in the model will consist of the study group assignment, the potential effect modifier of interest and the interaction between these two factors. Significance will be assessed by the p value for the interaction term, with values under 0.10 indicating a possible interaction and values under 0.05 confirming an interaction. Subgroups from categorical variables will be shown as a forest plot. Continuous variables will be analysed using cubic splines with 3–5 knots. If needed for data presentation, continuous variables will be dichotomised for inclusion in a forest plot. Prespecified subgroups that may modify the effect of the study group on the primary outcome (table 5).
Subgroup analyses of the primary outcome: successful intubation on the first attempt
Body mass index ≥30 kg/m2.
Sequential Organ Failure Assessment score ≥10.
Rank of the operator (attending anaesthesiology >5 years, attending anaesthesiology ≤5 years and resident).
MACOCHA score (0–3, 4–7 and 8–12)
Skill level of anaesthesiologist making first intubation attempt.
Number of previous intubation performed with videolaryngoscope (>50, 20–50 and <20).
Number of previous intubation performed with hyperangulated videolaryngoscope (>50, 20–50, <20).
Number of previous intubation performed with Macintosh videolaryngoscope (>50, 20–50 and <20).
Saturation oxygen <80% at the moment when intubation is decided.
Indication for intubation (acute respiratory failure vs other).
Presence of difficult airway characteristics as fixed effects (≥1 vs none).
Multivariable modelling to account for confounding.
Subsequent secondary analysis involving adjustment for prespecified baseline variables will be undertaken by fitting a regression mixed model for the primary outcome (successful intubation on the first attempt) as the dependent variable. Study site and operator will be considered as random effects, and study group (hyperangulated videolaryngoscopy or Macintosh videolaryngoscopy). Continuous variables will not be dichotomised for analysis but could be dichotomised for data presentation. We will present as RR and 95% CI.
Sample size calculation
We aim to compare the efficacy of first-attempt intubation in a critically ill patient using a hyperangulated blade compared with the efficacy achieved with a Macintosh blade. The efficacy percentage for the Macintosh blade is estimated at 84.0% (p1=0.84), and for the hyperangulated blade, it is 90.0% (p2=0.90). The sample proportion in each group will be balanced at a 1:1 ratio (w=0.50). Considering a significance level of 5.00% (α=0.05), in order to achieve a power of 80.00% (β=0.80) to detect significant differences, it will be necessary to recruit 492 patients in the hyperangulated blade group and 492 in the Macintosh blade group. Assuming a drop-out rate of 5% (ab=5%), a total of 1036 patients need to be recruited for the study. This justification has been carried out using statistical software ene3.0 for sample size calculations.
Monitoring
Data monitoring
Before the start of the study, all anaesthesiologists in the 20-night hospitals attended formal training sessions on the study protocol and data collection in the CRF. All documents required for the study are available for each researcher. Medical researchers oversee patients screening and inclusion. Data are collected in a web-based eCRF by trial personal. The eCRF is provided and managed by the Research Methodology Unit (Fundación Instituto de Investigaciones Sanitarias (FIDIS)) of Santiago de Compostela (Spain). Each patient receives a unique trial identification number. Only the investigators and research team have access to any protected health information of study participants and any study data. The principal investigators (MT, AE-G, JF and OV) meet with the principal investigators of each hospital to discuss any problems with data collection and protocol compliance and to evaluate study progress.
Harms
The study may be temporarily stopped for an individual patient, at the discretion of the attending physician, in case of major serious adverse events (SAEs) suspected to be associated with the type of videolaryngoscope used. Reporting of SAEs will be per the standard operating procedures of the Ethics Committee of Galicia (CEI). SAEs will include the following when occurring as a result of airway manipulation (eg, cardiac arrest, death, vocal cord injury and oesophageal rupture). The principal investigator will inform the CEI about the SAEs. No specific reporting procedure for unexpected serious adverse events is planned because strategies used in the two groups studied are standard care in the 30 hospitals of the study.
Auditing
The Research Methodology Unit (FIDIS) of Santiago, reviews the screening forms and clinical data at regular intervals.
Limitations of the study
The main limitation is that the study is not blinded. The nature of the trial intervention did not allow blinding of the operators or observers.
Ethics and dissemination
Research ethics approval
This study is conducted in compliance with the current version of the Declaration of Helsinki and Good Clinical Practice guidelines. The research project was approved by the Ethics Committee of Galicia (CEImG), Galicia, Spain (code No. 2024/031). The INVIBLADE-ICU trial was registered into the ClinicalTrials.gov clinical trials registry with ClinicalTrials.gov Registry: No. NCT06322719.
Consent or assent
Written informed consent is mandatory and will be obtained from all patients or their next of kin as required by the Ethics Committee of Galicia (CEImG). We will be able to use three methods to obtain written informed consent:
If possible, and the patient understands the information provided and is capable of responding, we will request written informed consent directly from the patient.
If the patient is unable to consent due to an underlying disease, they will be included after written informed consent is provided by a next of kin, if present.
If urgent intubation is necessary and no next of kin is present, the patient will be included in the study, randomised and data will be collected. Subsequently, consent will be sought from the next of kin. If the patient recovers from the condition that necessitated intubation, we will also seek their confirmation to participate in the trial.
A patient may leave the study at any time if the person informed about the study (patient or next of kin) is unwilling to continue in the study. Data from patients who request full withdrawal will not be considered in the analysis.
Patient and public involvement
The development of the research question and outcome measures was not informed by patients’ priorities, experiences and preferences. Patients were not involved in the design, recruitment and conduct of the study. The results will be available for study participants on demand.
Confidentiality
All original records will be archived on file at the trial sites for 15 years. The cleaned electronic trial database file will be anonymised and kept for 15 years.
Declaration of interest
None of the authors has any financial or other competing interests in relation to the study. The study is an investigator-initiated trial. The promotion of the study is carried out by 29 hospitals of Spain previously mentioned and the Health Research Institute of Santiago (IDIS). There is no industry support or involvement in the trial.
Protocol changes
Any additional amendments to the protocol will be documented on ClinicalTrials.gov in accordance with Standard Protocol Items: Recommendations for Interventional Trials guidelines.
Dissemination plan
Trial results will be submitted to a peer-reviewed journal and will be presented at one scientific conference.
Discussion
To the best of our knowledge, the INVIBLADE-ICU trial is the first randomised control study comparing successful intubation on the first attempt with the Macintosh videolaryngoscope versus the hyperangulated videolaryngoscope during tracheal intubation in critically ill patients. The standard Macintosh blades offer a lower angle of vision, but their advantage lies in their similarity to the blades commonly used in direct laryngoscopy, making them user-friendly for the person performing the tracheal intubation. Hyperangulated blades have a greater angle of vision, improving glottic visualisation, especially in patients with more complex airways.14 However, the need to overcome this angulation could potentially hinder the passage of the endotracheal tube to the vocal cords. It is unknown whether either blade could have an advantage for intubating critically ill patients.
After the study, we hope to have sufficient data to recommend the optimal blade for intubation with a videolaryngoscope in critically ill patients.
Trial status
Enrolment began on 1 May 2024 and is expected to be completed in 2025.
Ethics statements
Patient consent for publication
Acknowledgments
The authors wish to thank all anaesthesiologists from the study sites for their great effort and support of this trial.
Footnotes
X @Inma_F_Villa, @raferga73, @crisigleanest1
Contributors Guarantor of the study: MT. Study concept and design: MT, AE-G, JF, OV and TC. Adquisicion of data: MT, JF, APajares, FR, SM, IV, APérez, PR-M, MB, MP, RG-A, IF-V, JLA, MC, RC, AR, MMW, RF-G, AB, MG, FJG, MCI, RMS, SDV, LMC, MCA, IMR, MVarela, JIH and MVives. Analysis and interpretation of data: MT, AE-G and JF. Drafting of the manuscript and study supervision: MT, AE-G, JF, OV and TC. Approval of the final version of this manuscript and critical revision of the manuscript for important intellectual content: MT, AE-G, JF, OV, TC, APajares, FR, SM, IV, APérez, PR-M, MB, MP, RG-A, IF-V, JLA, MC, RC, AR, MMW, RF-G, AB, MG, FJG, MCI, RMS, SDV, LMC, MCA, IMR, MVarela, JIH and MVives.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.