Article Text
Abstract
Objective Hypertrophic cardiomyopathy (HCM), including obstructive HCM (oHCM), is the most common inherited cardiomyopathy causing lifestyle-limiting symptoms. Data are lacking about patients’ perspectives on the daily impact of their symptoms. This qualitative interview study was conducted to better understand patients’ experiences with oHCM.
Methods In October 2019, telephone interviews were conducted with 20 US adults with oHCM identified by the Hypertrophic Cardiomyopathy Association. Using a semi-structured interview guide, key symptoms, impacts of oHCM and oHCM treatment goals were discussed.
Results Median age was 54 years (range 29–78), 55% were women, 85% were white and 15% were Hispanic or Latino. Median time since diagnosis was 3 years. Symptoms included shortness of breath, dizziness/light-headedness, heart palpitations/fluttering (all 95%), fatigue (90%) and chest pain/pressure (80%). All participants reported limitations in physical functioning/activities; most reported additional impacts (emotional stress (80%), fear of dying (55%)). Shortness of breath and fatigue were among their most bothersome symptoms; an effective oHCM treatment would need to improve ≥1 of these symptoms (allowing increased physical/social activity).
Conclusions Patients with oHCM experience a high symptom burden and psychosocial impacts, affecting health status. Improved shortness of breath, fatigue and physical functioning are highly valued by patients and represent important treatment goals.
- cardiology
- quality of life
- cardiomyopathy
- patient reported outcome measures
- qualitative research
Data availability statement
Data sharing not applicable as no data sets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This study is one of the first to report patient experiences with obstructive hypertrophic cardiomyopathy using semi-structured, qualitative interview techniques.
The standardised, semi-structured interview guide provided patients with the opportunity to provide open-ended feedback and broaden our findings, while still ensuring consistency across interviews.
By using both a thematic analysis approach and descriptive statistics, we were able to capture both qualitative findings and quantitative data to support our conclusions.
Our sample size achieved thematic saturation and was appropriate for conducting detailed qualitative inquiries; however, it may limit the generalisability of the results.
Introduction
Hypertrophic cardiomyopathy (HCM) is characterised by left ventricular hypertrophy and is associated with lifestyle-limiting symptoms. HCM is the most common inherited cardiomyopathy, with an estimated prevalence of between 1 in 200 and 1 in 500.1 2 The main types of HCM are obstructive and non-obstructive HCM. Around 70% of patients have obstructive HCM (oHCM), which is characterised by resting or dynamic left ventricular outflow tract obstruction.3
Although oHCM is the most common inherited cardiomyopathy, patient experience with the disease is not well documented. As hard endpoints such as cardiovascular death and hospitalisations are infrequent among patients with oHCM, primary endpoints in clinical trials have included objective and subjective measures of functional capacity that are incomplete measures of health status.4–9 For example, the HCM Symptom Questionnaire assesses oHCM symptoms but does not impact health status10; the Kansas City Cardiomyopathy Questionnaire was developed for heart failure and does not account for symptoms unique to oHCM (eg, chest pain and dizziness/light-headedness)11; and the New York Heart Association classification is not only ascertained by physicians rather than patients but comprises only four categories. Furthermore, the daily variability of symptoms in oHCM is difficult to capture with existing tools. Therefore, there is a need to develop additional tools to strengthen health status assessment for patients with oHCM in clinical trials and real-world studies.
The first step in developing a sensitive measure of treatment effect on health status, to benefit the development and refinement of new therapies, is to obtain patients’ input on how oHCM impacts their lives. Understanding patients’ experiences with oHCM will also help inform which components need to be captured in standardised clinical history taking, help tailor treatment plans to specific patient needs and help identify system-level interventions that may be beneficial. Accordingly, our study team conducted a qualitative interview study aimed at characterising the symptoms, impacts and treatment goals of patients with oHCM.
Methods
Study design
In October 2019, RTI Health Solutions conducted in-depth, semi-structured, qualitative, one-on-one telephone interviews with 20 adults with oHCM. These interviews were designed to elicit a comprehensive set of oHCM symptoms and to explore how symptoms impact patients’ lives. The study sponsor, in collaboration with RTI Health Solutions, designed the interviews. RTI Health Solutions collected, analysed and interpreted the data.
Study population
The target study population comprised English-speaking adults with oHCM who were aged 18–65 years and resided in the USA. Inclusion criteria were left ventricular outflow tract (LVOT) peak pressure gradient ≥30 mm Hg at rest or with a provocation on echocardiogram; receiving treatment at a Hypertrophic Cardiomyopathy Association (HCMA)–designated HCM centre of excellence; and had at any time experienced at least one of the following prespecified symptoms: resting or exertional shortness of breath, heart palpitations or fluttering, dizziness or light-headedness, chest pain or pressure, fainting, weakness, fatigue and/or swelling in lower extremities. Exclusion criteria were prior septal reduction therapy (surgical myectomy or percutaneous alcohol septal ablation), coronary artery disease, persistent atrial fibrillation, chronic obstructive pulmonary disease, asthma or active malignancy for which they were receiving treatment.
Participant recruitment
Patients with oHCM who met enrolment criteria were identified by the HCMA. After reviewing their membership database, the HCMA invited 150 potential participants to undertake a screening survey to further evaluate eligibility. At this time, patient permission was also sought by the HCMA to share specific demographic information, clinical characteristics and contact information (name, telephone number, email) with RTI Health Solutions to facilitate interview scheduling. After reviewing the screening survey responses, the HCMA identified 20 adults with oHCM who met all inclusion and exclusion criteria using purposive, maximum variation sampling. Purposive sampling is a type of non-probability sampling where individuals meeting certain criteria are eligible for enrolment. Maximum variation is a type of purposive sampling where individuals differing in demographic and clinical characteristics are recruited to obtain varying perspectives.12 A sample size of 20 was prespecified to achieve thematic saturation (the point at which new themes are no longer reported and the same themes appear repeatedly) based on the concept of information power (the more information the sample holds relevant to the study, the fewer participants are needed) and on experience with prior qualitative, concept elicitation studies in other chronic diseases.13 14
Interview process and data collection
A standardised, semi-structured interview guide was used to ensure consistency across interviews (online supplemental table S1). The interview guide comprised an initial, open-ended, concept elicitation phase where participants were asked to describe their oHCM symptoms in detail and how they impacted their lives. Participants were then asked more targeted questions (probes; online supplemental table S2) to ensure the capture of a comprehensive set of symptoms and functional impacts, as well as identification of the relative importance of these symptoms and impacts (eg, most bothersome symptoms). Participants were also asked to describe variability in symptoms and the extent of improvement they would need to experience before concluding that a therapy was effective.
Supplemental material
Each 60 min interview was conducted by two experienced qualitative researchers from RTI Health Solutions, with one researcher leading the discussion and the other taking field notes and ensuring coverage of all topics. Audio recordings were made of the interviews, and these were transcribed verbatim and de-identified for analysis. Participants received a US$100 gift card in appreciation for their time.
Qualitative data analysis
A thematic analysis approach15 was used to analyse the results of the interviews, aided by field notes (in Excel, Office 365 version) and interview transcripts. Specifically, dominant themes were identified in each interview and compared across interviews to identify trends in the way participants described their experiences with oHCM (symptoms and impacts), as well as in the types of improvements they said they need to see before concluding that a treatment was effective. Additionally, descriptive statistics pertaining to demographic and clinical information were computed using Excel, Office 365 version. Both the qualitative and quantitative data were analysed and summarised in aggregate for the overall sample. No separate analyses by sex or gender were conducted.
Patient and public involvement
Patients were involved in the conduct of this study, and dissemination of study results. A patient-led organisation, the HCMA, conducted participant recruitment; and patients were able to provide input on interview content, due to the semi-structured nature of the interviews. The involvement of a patient author in the preparation of this manuscript enabled patient involvement in the dissemination of study findings. Results from this study will be used to develop a unique patient experience programme to capture data on all domains of quality of life―disease burden, treatment burden, psychological and emotional aspects, physical limitations and impact on work, social life and lifestyle.
Verbal informed consent was obtained prior to all telephone interviews.
Results
Baseline characteristics
Among the 20 participants, 11 (55%) were women. Median age was 54 years (range 29–78), and 17 (85%) participants were white (online supplemental table S3). Participants were distributed across the USA (Northeast (30%), Midwest (10%), South (45%) and West (15%)). Median time since oHCM diagnosis was 3 years (range 0.6–22).
Symptoms of oHCM
During screening, all participants (N=20) reported fatigue at rest and shortness of breath with activity (online supplemental figure S1). Other commonly reported symptoms included heart palpitations or fluttering, dizziness or light-headedness and weakness.
During the interview, nearly all participants reported experiencing shortness of breath, dizziness or light-headedness and heart palpitations or fluttering (n=19 (95%) for each; figure 1, table 1). Other common symptoms included fatigue (n=18 (90%)), chest pain or pressure (n=16 (80%)), swelling in lower extremities (n=10 (50%)), weakness (n=10 (50%)) and fainting (n=6 (30%)). Although not symptoms of oHCM, atrial fibrillation was spontaneously reported by seven participants and premature ventricular complexes by one participant. Shortness of breath (n=8) and fatigue (n=6) were most commonly included among participants’ most bothersome symptoms. Fewer participants reported dizziness or light-headedness (n=3), heart palpitations or fluttering (n=2), chest pain or pressure (n=2) or fainting (n=1) as one of their most bothersome symptoms.
Obstructive hypertrophic cardiomyopathy symptoms as reported by more than two interview participants, with representative quotes (N=20)
(Visual summary) All patient-reported symptoms and conditions mentioned during participants’ interviews (N=20). ‘Pain’ included pain in arms, jaw, neck and back. ‘Problems with cognition’ included difficulty concentrating or remembering things.
Although half of the participants reported that their symptoms were generally consistent from day-to-day, others reported that they were variable. One participant stated, ‘It changes based on diet, alcohol, sleep, the weather; any number of factors will change how I feel on a given day. I know when I’m doing something that I [will] pay the price [for]. If I really need to feel good … I know what to stay away from’.
Impact of oHCM on health status
Most participants mentioned physical limitations, with the most common being walking (n=18), climbing stairs (n=15) and lifting (n=10) (table 2). The severity of physical limitations varied among participants; for example, although a few reported that they were unable to walk as far as they would like, some had difficulty walking even short distances. Participants commonly reported limitations in their daily activities due to symptoms of oHCM, such as household chores (n=18), recreational activities (n=18), social activities (n=13), eating (n=11), exercise (n=11) and working (n=9).
Impact of obstructive hypertrophic cardiomyopathy on patient-reported health-related quality of life, as described by interview participants (N=20)
Many participants reported emotional burden related to associated stress, anxiety and a sense of isolation due to their oHCM (n=16). More than half (n=11) declared they fear dying, and half (n=10) reported they have difficulty keeping or nurturing relationships with family and friends. Limitations in physical functioning or activities (n=8) and emotional impact (n=5) were cited as having the most negative impact on participants’ overall quality of life. Participants also reported that having specific symptoms (n=6) or the ability to maintain social relationships (n=4) greatly affected their quality of life.
Expectations of treatment
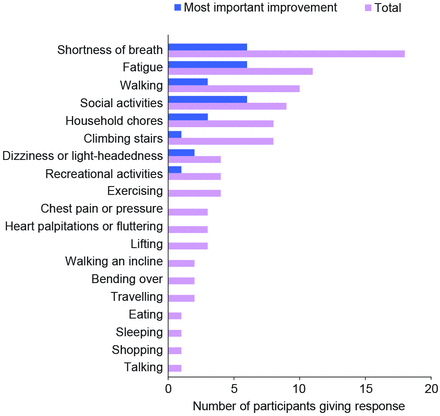
When asked what would make them consider a new oHCM treatment to be effective, almost all participants (n=18) stated they would need to see an improvement in shortness of breath and more than half (n=11) would have to experience improvement in fatigue (figure 2, table 3). Fewer participants reported a need for improvements in dizziness or light-headedness (n=4), chest pain or pressure (n=3) or heart palpitations or fluttering (n=3) for a treatment to be considered effective. In addition, some participants said they would need to see improvements in walking (n=10), climbing stairs (n=8), participating in social activities (n=9) and performing household chores (n=8). Participants consistently explained that improvement in their shortness of breath and fatigue would correlate with enhanced ability to perform these physical and social activities.
Patient expectations from therapy for obstructive hypertrophic cardiomyopathy (N=20)
Improvements that indicate effective treatment, as reported by interview participants (N=20).
Discussion
This study is one of the first to report patient experiences with oHCM using semi-structured, qualitative interview techniques. Three key findings have emerged. First, shortness of breath and fatigue were the most commonly reported symptoms and most frequently identified as participants’ most bothersome symptoms. Second, participants commonly described significant impacts on their psychosocial functioning that were not solely due to physical limitations. Finally, participants reported that reductions in their burden of shortness of breath and fatigue, accompanied by improvements in their ability to engage in routine social and physical activities (eg, walking and climbing stairs), were necessary for them to consider a treatment effective.
Our study confirms and extends prior reports on the value of improved functional capacity and symptom relief for patients with oHCM or similar conditions. In a similar study, Zaiser et al described qualitative health-related experience among patients with oHCM and non-obstructive HCM and revealed the most frequent and most important symptoms were shortness of breath, palpitations, fatigue/tiredness, dizziness/light-headedness and chest pain.14 Our findings represent a US-based population and extend this previous work by Zaiser et al by including a detailed qualitative inquiry into treatment goals from a patient perspective. All participants in our study had oHCM, whereas the study by Zaiser et al enrolled patients with oHCM and non-obstructive HCM. As such, the concepts raised in our study represent important treatment goals for patients with oHCM specifically. The importance participants in our study place on improvement in symptoms and function as treatment goals is consistent with a survey of patients with heart failure by the US Food and Drug Administration and Heart Failure Society of America. In this survey, 26.2% of respondents would be willing to risk having less time alive to improve how they feel or function.4 Our results suggest that, as for patients with heart failure, increasing time alive is not the only treatment goal for patients with oHCM and survival is coupled with the need for symptom reduction and improved quality of life across multiple domains.
Findings from our study underscore the high prevalence of both physical and psychosocial symptoms and the resulting impaired health status of patients with oHCM. This finding may inform the development of tools to measure patient-reported outcomes (PROs) that are specific to oHCM. Participants in our study frequently reported psychosocial impacts, including emotional distress and fear of dying and negative impacts on relationships with family and friends. Accordingly, a thorough evaluation of oHCM impacts should include PRO tools assessing the severity and frequency of shortness of breath, fatigue, chest pain or pressure and dizziness or light-headedness, as well as tools evaluating physical activity, psychosocial and quality-of-life impacts. Future long-term programmes should also be developed to capture a broad range of patient experience data covering all domains of health-related quality of life―disease burden, treatment burden, psychological and emotional aspects, physical limitations and impact on work, social life and lifestyle. Development of improved PRO tools specific for oHCM may support the adoption of symptom relief and improved functional capacity as regulatory endpoints for oHCM treatments, as is already recommended for heart failure treatments in the USA.16 These assessments could then be implemented in randomised controlled trials of novel therapies, enabling symptom relief and functional benefit to become a focus for drug development.
The results of our study should be considered in light of several limitations. First, the generalisability of results may be limited by the small number of participants and possible selection bias during participant recruitment. The eligibility criteria included patients with resting or exertional shortness of breath, heart palpitations or fluttering, dizziness or light-headedness, chest pain or pressure, fainting, weakness, fatigue and/or swelling in lower extremities and thus resulting in patients reporting symptoms in this study. Additionally, as the prevention of sudden cardiac death has been a priority focus of the HCMA, patients reporting fear of dying could present possible selection bias. To minimise these impacts, we used purposive, maximum variation sampling to ensure diversity in participants’ demographic and clinical characteristics. Also, we achieved thematic saturation, and our estimated sample size is similar to that in previous qualitative and interview studies in the field of cardiac failure.12 17–20 In addition, the small sample size enabled us to conduct detailed qualitative inquiries.
Second, due to confidentiality requirements, we were unable to capture detailed demographic data and granular clinical data on the severity of oHCM, such as the degree of LVOT gradient elevation, extent of hypertrophy or severity of diastolic dysfunction. However, prior studies have shown symptoms in patients with oHCM do not necessarily correlate with LVOT gradient.21 The results of this study may also have been impacted by the wide variability in participants’ age and time since diagnosis. For example, comorbidities more common with increasing age might confound patient experiences with oHCM symptoms. Alternatively, patients with a longer time since diagnosis may become accustomed to their symptoms over time. Future research could investigate whether the impacts of oHCM on patients varies with age and time since diagnosis and evaluate the differences between patient symptom profiles and LVOT gradients, including those eligible for septal reduction therapy. Finally, we did not conduct any separate analyses by sex or gender; this was due to the small sample size.
In conclusion, patients with oHCM in this study experienced a high burden of symptoms and additional psychosocial impacts that led to poor health status. Improvements in shortness of breath, fatigue and physical functioning would be highly valued by patients with oHCM and represent important treatment goals for emerging HCM medications. The results of this analysis will be used to develop a unique patient experience programme to capture a broad range of data covering all domains of quality of life―disease burden, treatment burden, psychological and emotional aspects, physical limitations and impact on work, social life and lifestyle.
Data availability statement
Data sharing not applicable as no data sets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Ethics approval
Given the low-risk nature of this qualitative study, it was deemed exempt from formal review by RTI Health Solutions’ institutional review board. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We acknowledge Susan Tan, PhD, for editorial support, on behalf of Envision Pharma Group, Sydney, Australia, and funded by Cytokinetics, Incorporated.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors MB is responsible for the overall content as guarantor. CE, KK, SF and MB contributed to conceptualisation and methodology. SSh, LS, SBH, DJ and SSa contributed to validation. CE, KK and SF conducted the formal analysis. MB contributed to investigation and resources. CE, KK, SF and MB conducted the data curation. SSh, MB and SSa prepared the original draft. All authors reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding This study was funded by Cytokinetics, Incorporated (grant number not applicable). The article processing charge was funded by Cytokinetics, Incorporated. Employees of the funding organisation participated in the analysis and interpretation of data. Cytokinetics, Incorporated, was given the opportunity to review the manuscript for medical accuracy and intellectual property protection. The manuscript authors had control of the decision to submit the study results for publication.
Competing interests SSh has received a grant from the American Heart Association (grant ID 855105). CE, KK and SF are employees of RTI Health Solutions. LS has no conflicts of interest to declare. MB, SBH and DJ are employees of Cytokinetics, Incorporated, and hold stock in Cytokinetics, Incorporated. SSa has received consultant/adviser fees from Bristol Myers Squibb and research grants from Bristol Myers Squibb, Cytokinetics, Novartis and Actelion Pharmaceuticals.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.