Article Text
Abstract
Introduction We previously reported global regional differences in smoking cessation outcomes, with smokers of US origin having lower quit rates than smokers from some other countries. This post-hoc analysis examined global regional differences in individual-level and country-level epidemiological, economic and tobacco regulatory factors that may affect cessation outcomes.
Methods EAGLES (Evaluating Adverse Events in a Global Smoking Cessation Study) was a randomised controlled trial that evaluated first-line cessation medications and placebo in 8144 smokers with and without psychiatric disorders from 16 countries across seven regions. Generalised linear and stepwise logistic regression models that considered pharmacotherapy treatment, psychiatric diagnoses, traditional individual-level predictors (eg, demographic and smoking characteristics) and country-specific smoking prevalence rates, gross domestic product (GDP) per capita, relative cigarette cost and WHO-derived MPOWER scores were used to predict 7-day point prevalence abstinence at the end of treatment.
Results In addition to several traditional predictors, three of four country-level variables predicted short-term abstinence: GDP (0.54 (95% CI 0.47, 0.63)), cigarette relative income price (0.62 (95% CI 0.53, 0.72)) and MPOWER score (1.03 (95% CI 1.01, 1.06)). Quit rates varied across regions (22.0% in Australasia to 55.9% in Mexico). With northern North America (USA and Canada) as the referent, the likelihood of achieving short-term abstinence was significantly higher in Western Europe (OR 1.4 (95% CI 1.14, 1.61)), but significantly lower in Eastern Europe (0.39 (95% CI 0.22, 0.69)) and South America (0.17 (95% CI 0.08, 0.35)).
Conclusions Increased tobacco regulation was associated with enhanced quitting among participants in the EAGLES trial. Paradoxically, lower GDP, and more affordable cigarette pricing relative to a country’s GDP, were also associated with higher odds of quitting. Geographical region was also a significant independent predictor.
Trial registration number ClinicalTrials.gov, NCT01456936.
- health policy
- international health services
- health economics
Data availability statement
Data are available upon reasonable request. Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
EAGLES (Evaluating Adverse Events in a Global Smoking Cessation Study) is the largest randomised, placebo-controlled trial of cessation medications that enrolled persons with and without psychiatric disorders who smoke in 16 high-income and middle-income countries across five continents.
The present post-hoc analysis of EAGLES trial results extends prior work by incorporating novel country-specific and region-specific factors as predictors of smoking cessation outcomes.
The EAGLES trial was not designed to recruit representative samples of a country’s smokers; but rather, to enrol smokers who met prespecified inclusion/exclusion criteria, which may limit generalisability.
Introduction
An estimated 1.3 billion (roughly 1 in 5) people worldwide use tobacco.1 Although global smoking prevalence is decreasing,2 the number of smokers continues to increase.2 Smoking is the leading cause of preventable death worldwide.3 Tobacco-related deaths are increasing,2 with more than 8 million deaths per year attributable to tobacco.1
As of 2017, high-income countries still had higher smoking prevalence rates (21.6%) than low-income (11.2%) and middle-income (19.5%) countries.4 However, high-income countries also show disproportionately greater reductions in smoking prevalence than low-income and middle-income countries.5 As a result, low-income to middle-income countries are now home to 80% of the world’s population of smokers1 and report the majority of tobacco-related deaths.6
Smoking prevalence also varies greatly by geographical region. According to the WHO prevalence estimates for 2015, the European region had the highest smoking rates (29.9%), followed by the Western Pacific region (24.8%); the African region had the lowest (10.0%).4 Although smoking prevalence is decreasing (and expected to continue decreasing) in most regions, the eastern Mediterranean is projected to be an exception.6
In 2003, to address these disparities, the WHO established the Framework Convention on Tobacco Control (FCTC), which outlines policies and measures to promote tobacco use prevention and treatment globally.7 To track the progress of individual countries, WHO developed a quantitative measure—the MPOWER score. This grades a country’s tobacco control efforts across six domains (table 1). Countries with higher MPOWER scores showed a greater reduction in smoking prevalence over the first decade of FCTC implementation.8 However, regional disparities in overall tobacco use prevalence cannot be fully addressed without understanding the contributors to such disparities, specifically whether these could also be influencing regional cessation rates. Individual-level predictors of smoking cessation are widely studied in the literature. Fewer studies have explored how country of origin might influence abstinence. The International Tobacco Control Four Country Survey (ITC-4) was a large prospective cohort study that involved telephone surveys of more than 2000 smokers in Australia, Canada, the UK and the USA. An analysis of the ITC-4 data by Hyland et al9 demonstrated that these countries’ smoking cessation rates were not equally moderated by traditional individual predictors such as the Heaviness of Smoking Index, and favourable attitudes about smoking and self-efficacy for quitting. Furthermore, heaviness of smoking was associated with lower income in all countries but the USA.10
Country-level economic, epidemiological, and policy variables
Our prior work similarly noted regional effects on smoking cessation rates, while also incorporating the impact of pharmacotherapy. One secondary analysis of a study examining the effect of varenicline on depressed smokers demonstrated that European participants were four times more likely to achieve abstinence than US participants, and that higher levels of baseline depressive symptoms were associated with lower abstinence rates for European but not US participants.11
One proposed explanation for these results is the ‘hardening hypothesis’—that areas with lower smoking prevalence are composed of more ‘hardened’ smokers who have greater difficulty quitting. Smokers who found it easier to quit have already quit, and the remaining hardened smokers are more nicotine dependent, of lower socioeconomic status and have a greater likelihood of psychiatric comorbidity.12 This hypothesis has been difficult to consistently support.12–14 A major gap within the ‘hardening’ literature is that most studies have been conducted in high-income countries.12 If hardening were to be demonstrated on a broader global scale, there could be significant implications for international tobacco policy.
Similar limitations exist in the literature on predictors of smoking cessation: regional differences are primarily examined among high-income, Westernised countries. Fewer studies include geographically and economically diverse countries. Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES) was a large-scale, multinational, randomised, placebo-controlled, smoking cessation pharmacotherapy study, conducted from 2011 to 2015, that offered a unique opportunity to examine smoking cessation outcomes on a global level.15 Participants were recruited from 16 high-income and middle-income countries across five continents. There were significant regional differences in smoking cessation outcomes,16 with lower abstinence rates in, compared with outside, the USA (even after controlling for other factors).
This paper explores these findings from EAGLES, as, to our knowledge, no large-scale randomised controlled trials have examined global regional differences in predictors of smoking cessation outcomes among both high-income and middle-income countries. Our first aim was to examine regional demographic, smoking and psychiatric differences, and we hypothesised that significant baseline differences would be observed across regions. Our second aim was to explore whether region-specific and country-specific variables—such as income, cigarette affordability, prevalence of tobacco use and tobacco control policy—were associated with cessation outcomes. We hypothesised that participants from countries with more proactive tobacco control policies would have a less robust response to smoking cessation interventions than their counterparts due to possible ‘hardening’.
Methods
Design
This is a secondary analysis of data collected from the randomised, double-blind, triple-dummy, EAGLES trial (ClinicalTrials.gov), which investigated the safety and efficacy of varenicline (1 mg two times daily) and bupropion (150 mg two times daily) in an active-controlled (nicotine patch, 21 mg/day) and placebo-controlled study in 8144 smokers with (n=4116) and without (n=4028) psychiatric disorders. Participants received 12 weeks of active treatment (or placebo) and were followed for an additional 12 weeks, and all participants received brief cessation counselling. The primary outcome paper includes further details about the study methodology and follows reporting recommendations set out by Consolidated Standards of Reporting Trials guidelines.15 17 Briefly, eligible participants were stratified into a non-psychiatric cohort (NPC) and four subcohorts (see below) in the psychiatric cohort (PC) based on their primary psychiatric diagnosis, and by site region across four prespecified geographical zones (USA, Western Europe and Other Countries, Eastern Europe and South and Middle America). Treatment groups were balanced across the five diagnostic groups for each of the four regions. A computer-generated randomisation schedule was used to assign participants to treatment using a block size of eight (1:1:1:1 ratio) for each of the diagnoses by region combinations.
Participants
Participants were male and female smokers, aged 18–75 years, who were motivated to quit smoking and smoked, on average, ≥10 cigarettes per day. Those in the PC met Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV-TR)18 criteria for either (1) a mood disorder (major depressive or bipolar disorders); (2) an anxiety disorder (panic, post-traumatic stress or obsessive-compulsive disorder, social phobia or generalised anxiety disorder); (3) psychotic disorder (schizophrenia or schizoaffective disorder); or (4) borderline personality disorder as confirmed by the Structured Clinical Interview for the DSM-IV-TR for Axis I/II disorders (SCID-I/II).19 20 Participants in the NPC had no history of mental illness, as confirmed by SCID-I/II. For this secondary analysis, we grouped countries into seven regions based on their geographical proximity and similarities in demographic characteristics (table 2).
Country-specific variables by region
Primary outcome measure
The primary outcome for this secondary analysis was 7-day point prevalence abstinence (PPA) at the end of treatment (week 12) defined as self-reported no smoking for 1 week confirmed by expired breath carbon monoxide levels <10 parts per million at that study visit. This endpoint was selected to amplify the abstinence signal as early abstinence has been shown to strongly predict future long-term abstinence.21
EAGLES independent variables
Participant characteristics associated with continuous abstinence from 9 to 24 weeks were included as candidate predictor terms in this secondary analysis.16 These included age, gender, body mass index (BMI), race (white vs non-white), nicotine dependence severity (measured by Fagerström Test for Cigarette Dependence),22 cigarettes per day in the month prior to enrollment, prior use of smoking cessation medications (varenicline, bupropion or nicotine replacement therapy (NRT)), age when started smoking, lives with smoker and has contact with smokers. Additionally, we included seven mental health characteristics: comorbid psychiatric diagnosis (none, mood disorder, anxiety disorder, psychotic disorder)18; depression symptom severity (measured by Hospital Anxiety and Depression Scale (HADS))23; anxiety symptom severity (measured by HADS)23; aggression symptom severity (measured by Buss–Perry Aggression Questionnaire)24; lifetime suicidal behaviour and/or ideation (yes/no, measured by Columbia–Suicide Severity Rating Scale)25; comorbid alcohol or other substance dependence (defined by DSM-IV-TR and confirmed by SCID-I/II)18; and use of psychotropic medication (yes/no).
Non-EAGLES country-level independent variables
Four country-specific variables were sourced to reflect their values during the period in which EAGLES was conducted (2011–2015) (table 1).
Baseline tobacco smoking prevalence was extracted from WHO statistics on smoking prevalence rates from 2015.5 To measure the regional economic influence on cessation outcomes, both absolute and relative measures were obtained. The gross domestic product (GDP) of each country was measured as GDP per capita in US dollars in 2014 (as reported by the World Bank),26 which was then divided by 10 000 to facilitate effect interpretation. To look at the affordability of cigarettes in a country, we use the ‘relative income price’ (RIP) measure, calculated as the percentage of GDP per capita required to purchase 2000 cigarettes (100 packs) of the most sold brand (data from 20145).
The rigour of each country’s tobacco control policy was estimated using the WHO’s 37-point MPOWER score, which quantifies the degree of implementation and enforcement of the FCTC. Points are awarded according to six core domains (table 1).27 A higher score indicates greater adherence to FCTC guidelines, with a maximum possible score of 37. Table 2 illustrates the country-level variables (tobacco prevalence, GDP, cigarette RIP and MPOWER score) we derived for all 16 countries in which EAGLES participants were enrolled. It further depicts the seven geographical regions we characterised to capture these regional differences. Each EAGLES participant was assigned values for these four variables corresponding to the location of their respective study site.
Statistical analysis
Descriptive statistics were compiled to examine baseline differences by country and geographical region, with respect to demographic, smoking and mental health characteristics. A correlation assessment for the country-level variables was reviewed to alleviate any multicollinearity concerns with these measures. For the primary efficacy endpoint of 7-day PPA at week 12, model building used a stepwise, logistic regression analysis. Significance levels were set a priori as 10% for a variable to enter and 15% to remain in the model. The method forced the inclusion of treatment condition (placebo, varenicline, bupropion, NRT) and cohort (PC and NPC). Main-effect candidates included regions (7-level), four country-level non-EAGLES variables and 17 EAGLES baseline characteristics, described above. All randomised subjects were included, with ORs (95% CIs) computed.
Patient and public involvement
None.
Results
Smoking prevalence rates varied widely across the countries and regions represented in EAGLES (table 3). Smoking rates were highest in the Russian Federation and Eastern Europe. Australia, Brazil, Canada and Mexico had smoking prevalence rates below 15%. There was also marked variability in countries’ GDP, with Denmark and Australia registering as the highest-income countries, and South Africa and Bulgaria as the lowest among EAGLES countries. A relative cost of cigarettes was highest in South Africa and Bulgaria; the USA had the lowest cigarette RIP in 2014. MPOWER scores ranged from a low of 14 in South Africa to a high of 34 in Brazil. These four variables were not significantly correlated (data not shown).
Baseline characteristics by region (demographic, smoking, psychiatric and country-level variables)
Mean tobacco smoking prevalence was highest in Eastern Europe (32.8%) and tied for lowest in Australasia and North America II (Mexico) (15.0%). Although North America II (Mexico) had the lowest proportion of participants with psychiatric diagnosis and no active substance use disorders, participants enrolled in this country had the highest baseline levels of anxiety (5.8±4.1), depression (3.7±3.2) and aggression (62.2±17.8) scores. South Africa had the lowest GDP per capita (6433±0.0) and lowest MPOWER policy score (14.0±0.0). South America had the highest MPOWER score (32.8±1.1).
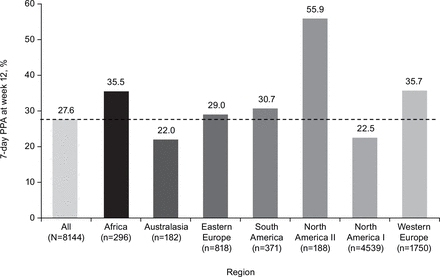
Seven-day end-of-treatment PPA varied widely across regions (figure 1), with the lowest rates found in Australasia (22.0%) and North America I (22.5%) and the highest rate (55.9%) in North America II (Mexico).
Seven-day PPA at week 12 by region. All patients randomised. PPA, point prevalence abstinence.
Table 4 depicts the results of the stepwise regression model examining the association of the 17 candidate predictor variables and the primary endpoint of 7-day PPA. Consistent with prior analyses of EAGLES data, individuals of black compared with white race (OR 0.622 (95% CI 0.518, 0.748)), with psychotic disorders (0.605 (95% CI 0.435, 0.841)), psychiatric medication use (0.789 (95% CI 0.688, 0.904)), more cigarettes per day (0.968 (95% CI 0.960, 0.976)) and contact with a smoker (0.856 (95% CI 0.764, 0.961)) had lower odds of achieving short-term abstinence. Higher abstinence rates were observed in older participants (OR 1.010 (95% CI 1.005, 1.014)), with greater BMI (1.013 (95% CI 1.004, 1.022)) and with prior varenicline use (1.228 (95% CI 1.060, 1.422)). Additionally, all treatment groups demonstrated higher odds of abstinence as compared with placebo, as follows: varenicline (OR 3.808 (95% CI 3.260, 4.447)), bupropion (2.059 (95% CI 1.755, 2.417)) and NRT (2.103 (95% CI 1.793, 2.468)).
Main-effect ORs for final stepwise logistic regression model of 7-day PPA, week 12
After controlling for those traditional predictor variables, the region remained in the model as a significant main effect. Using North America I (USA and Canada) as the referent, the odds of achieving short-term abstinence were significantly higher in the Western European (OR 1.356 (95% CI 1.140, 1.613)) and lower in the Eastern European (0.390 (95% CI 0.222, 0.686)) and South American (0.170 (95% CI 0.083, 0.348)) regions.
Of the four country-level variables, three predicted abstinence (table 4). Lower odds of abstinence were seen with higher GDP (OR 0.544 (95% CI 0.468, 0.631)) and higher cigarette RIP (0.617 (95% CI 0.528, 0.722)), whereas higher odds were seen with higher MPOWER score (1.031 (95% CI 1.008, 1.055)). Notably, tobacco smoking prevalence was not included in the model.
Discussion
As predicted, individual-level variables of demographic, psychiatric and smoking-related characteristics, as well as country-level variables of income, cigarette relative income price and implementation of tobacco control policy, were associated with the likelihood of quitting. Specifically, the higher the income of a country and the more expensive cigarettes relative to a country’s per capita GDP, the lower the likelihood of abstinence at the end of treatment. Conversely, more stringent tobacco control policy implementation was associated with increased rates of abstinence. Finally, country-level tobacco prevalence at the time the EAGLES study was conducted was not significantly correlated with abstinence initiation rates. After controlling for these and other traditional predictor variables, the global region was still found to be a significant independent predictor of short-term smoking abstinence.
Despite adhering to the same study protocol with standardised inclusion and exclusion criteria used to enrol smoking participants, baseline characteristics by region differed broadly across the board with respect to age, gender, race, psychiatric history, psychiatric symptoms, prior treatments, severity of tobacco use and dependence and substance use history. For instance, participants enrolled in the South American region were the oldest, smoked the most cigarettes per day and were 99% white; Africa was the only region where males predominated and participants were predominantly non-white. Some regions had a substantial number of participants who had previously tried smoking cessation treatments, but regions such as Eastern Europe and North America II (Mexico) had hardly any. These individual-level characteristics have been shown to be independently associated with tobacco cessation outcomes, both in our earlier analysis16 and in the literature more generally.11 21 28 There is a growing body of literature suggesting the benefit of interventions specific to these risk factors,29–31 and one might extrapolate a potential benefit in tailoring a region’s tobacco control plan to its unique characteristic makeup.
We found that a greater degree of tobacco control policy implementation, as reflected by higher MPOWER scores, was associated with higher odds of achieving short-term abstinence in EAGLES. This suggests that greater tobacco regulation is associated with higher quit rates, which is corroborated by the literature32 and aligns with the greater mission of the FCTC. Although it may be presumed that greater tobacco control would be found in higher-income regions and reflected by higher-priced and taxed cigarettes, our analysis did not find that to be the case. In fact, not only did we not find a correlation between those variables, but we found an inverse relationship with cessation rates. Our analysis found that higher income and more expensive cigarettes (ie, higher RIP) were associated with lower cessation rates. This paradox comes as a surprise among the growing body of literature reporting that higher-income countries have had more drastic reductions in smoking prevalence,5 thought to be due to greater funding for and access to cessation interventions.33 However, a newer, large-scale global analysis, published by Sathish et al,34 found that smokers in high-income countries were consuming cigarettes with much higher levels of nicotine than those in middle-income or lower-income countries, which might make it harder to quit.34 The literature also supports the idea that increasing the price of cigarettes is associated with a greater likelihood of quitting,6 35 which is in opposition to our finding. But here again, as demonstrated in South Africa,36 raising prices on cigarettes via taxes may inadvertently lead to a proliferation of illicit cigarettes and the introduction of cheaper local brands, which may undermine tobacco regulatory efforts.
One possible explanation for these curious results is the controversial ‘hardening hypothesis’ that smokers who find it easier to quit have already done so, leaving ‘hardened’ smokers. If someone continues to smoke cigarettes despite the increasing cost, that individual may fall under the umbrella of a ‘hardened’ smoker, and thus have more difficulty quitting. The same may apply to higher-income regions, with presumed greater access to healthcare and cessation resources. However, hardening is commonly attributed to populations with lower smoking prevalence,12–14 and in our analysis, a region’s smoking prevalence rate at the time EAGLES was conducted was not a significant predictor of smoking cessation success once other variables were included in the model. Basing the hardening hypothesis purely on smoking prevalence at a single time point is likely too reductionist a model. For example, Cheung et al found a model that may unite contradictory findings about hardening.37 Their sample showed a U-shaped relationship between the odds of quitting smoking and smoking prevalence, in which the odds of quitting were highest at either extreme of the smoking prevalence curve.
Even though we examined these regional effects in a more granular, seven-region context compared with our prior EAGLES analyses, which considered only a US/non-US dichotomy, the region from which subjects were enrolled remained a significant main effect in the analytical model despite also controlling for treatment group and psychiatric subcohort. The EAGLES data set was not intended to represent the global population of smokers at large, nor was its enrollment strategy designed to randomise participants within each of the countries participating. Nevertheless, our regional findings appeared to have similar trends to others described in the literature. Our prior work11 did not make the distinction between Eastern and Western Europe, but found that European smokers had higher rates of abstinence overall compared with US smokers. In our current analysis, we found that, when compared with North American I (USA and Canada) participants, smokers enrolled in the Western European region had approximately one-third higher odds of abstinence, whereas enrollees in Eastern Europe had less than half the odds of quitting. The literature supports this finding, and when compared with Western Europe, Eastern Europe has been found to have lower smoking cessation rates,38 higher smoking prevalence rates and higher rates of morbidity and mortality attributable to tobacco.5 These challenges are thought to be due to more accessible cigarettes, less tobacco control and particular cultural and religious practices in the region.5 We also found that smokers enrolled at sites in South America had the lowest odds of successful cessation—about one-quarter of the odds in North America I (table 4). A 2008 review paper from Müller and Wehbe39 examined unique factors in Latin America that contribute to its growing tobacco epidemic, particularly that this region includes some of the highest tobacco-producing countries in the world (in our data set, Brazil #3 and Argentina #9), and that such an economic reliance on tobacco products has likely contributed to less rigorous tobacco control, less expensive cigarettes and an ongoing tobacco smuggling trade.39 It is curious then, in our analysis, that this region had the highest MPOWER score. Because our model was designed to include all regions, each predictor might not extrapolate to each individual region.
Our analyses were not without limitations. The EAGLES trial was not designed to recruit representative samples of a country’s smokers, but rather, to enrol smokers who met prespecified inclusion/exclusion criteria into a methodologically sound, randomised controlled trial comparing the first-line smoking cessation medications and placebo. Thus, the results might not generalise to the global population of smokers at large and may not be representative of each country’s smokers. Sites enrolling participants in EAGLES were located primarily in high-income and upper-middle income countries, further limiting generalisability. Over half the EAGLES participants were enrolled in the USA, an imbalance that could have affected results. Although we controlled for treatment condition and psychiatric cohort in our analyses and examined correlations among the newly introduced country-level variables, we cannot rule out multicollinearity among predictor variables affecting the results. Moreover, we did not assess how sociocultural factors, including differences in stigma levels surrounding reporting mental health conditions across countries, may have influenced results. Nonetheless, EAGLES remains the largest, most rigorous, placebo-controlled, multinational trial of smoking cessation medications ever conducted, and the new results obtained will help inform subsequent analyses in samples more representative of smokers across the globe.
In conclusion, geographical region had a significant effect on the odds of achieving short-term smoking abstinence in EAGLES even after controlling for treatment, psychiatric comorbidity, individual-level and country-specific variables. Increased tobacco control policy and enforcement was associated with a greater chance of achieving short-term abstinence, which supports the argument that tighter regulation is associated with enhanced efficacy of smoking cessation treatments. Although seemingly contradictory, increased income of a country and more expensive cigarettes were associated with lower odds of abstinence, which might reflect the hardening of smokers in those countries. The literature remains mixed about whether hardening truly exists; it may be that a deeper understanding of this complex phenomenon is needed, rather than refuting the validity of the hypothesis itself.
Data availability statement
Data are available upon reasonable request. Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics statements
Patient consent for publication
Ethics approval
The EAGLES study was a large multisite trial with no single overarching ethics approval board. Investigative sites were permitted to use either their own local institutional review boards (IRBs) or various central IRBs. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank Alok Krishen for his input on this manuscript. Data were previously presented in part at the Society for Research on Nicotine & Tobacco’s 27th Annual Meeting, February 2021. Editorial support was provided by Gemma Shay-Lowell, PhD, and Katy Beck, PhD, of Engage Scientific Solutions, and was funded by Pfizer.
References
Footnotes
Contributors AEE and RMA were involved in the conception and design of the parent study. BD, DEL, BSM, PS, TM, AEE and RMA were involved with the post-hoc analyses and/or interpretation of the data. DEL performed the statistical analyses. RMA and DEL are responsible for the overall content as guarantor. All authors were involved in the drafting of the manuscript and revising it critically for intellectual content, provided final approval of the version to be published and agree to be accountable for all aspects of the work.
Funding Pfizer and GlaxoSmithKline.
Competing interests BD has no funding sources to disclose. DEL and PS are employees and stockholders of Pfizer. TM has recently retired from Pfizer and is a stockholder. AEE has received editorial support from Envision Pharma, has served as a consultant to Charles River Analytics and to Karuna Pharmaceuticals, and is a founder of NirVue. RMA received research support from Pfizer and Embera NeuroTherapeutics. He provided consultancy to Pfizer Korea and has received editorial support from Envision Pharma funded by Pfizer. BSM has no funding sources to disclose.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.