Article Text
Abstract
Purpose To analyse retinal and choroidal microvasculature features in healthy pregnant women using optical coherence tomography angiography (OCTA).
Methods PubMed, Embase, Ovid, Web of Science and Cochrane Library were searched for published studies that compare retinal and choroidal microvasculature characteristics between pregnant females and non-pregnant females from inception to November 2023. The statistics were continuous variables. They were presented as the mean difference (MD) with a corresponding 95% CI. We used Review Manager software 5.4.1 for statistical analysis.
Results A total of 5 eligible studies involving 219 eyes in the pregnancy group and 186 eyes in the control group were included in the meta-analysis. The pooled results showed that the retinal deep capillary plexus vascular density (DCP VD) measured by OCTA was significantly higher in healthy pregnant women than in controls (MD 1.53; 95% CI 0.62 to 2.44; I2=0%; p=0.0009). However, differences between the two groups in the retinal superficial capillary plexus VD (SCP VD) (MD 0.41;95% CI −1.26 to 2.09; I2=85%; p=0.63), the foveal avascular zone (FAZ) (MD 0.01; 95% CI −0.01 to 0.03; I2=14%; p=0.18), the choriocapillaris VD (CC VD) (MD 0.76; 95% CI −1.11 to 2.64; I2=79%; p=0.43) were uncertain.
Conclusions Our meta-analysis found that the DCP VD of healthy pregnant women was higher than that of non-pregnant controls. However, differences in SCP VD, FAZ and CC VD between the healthy pregnant women and the non-pregnancy controls were uncertain. Our findings can help to get a deeper understanding of retinal and choroidal microvascular characteristics during pregnancy.
- Pregnancy
- Vetreoretinal
- OBSTETRICS
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study systematically reviewed the retinal and choroidal microvascular features during pregnancy.
We assessed the evidence quality using the Grading of Recommendations Assessment, Development and Evaluation approach, which provided essential information for primary outcomes.
The total sample size in the included studies was relatively small.
There exists heterogeneity across the included studies.
Introduction
Pregnancy is a physiological state which causes a variety of anatomic and functional body changes in several organ systems, such as metabolic, cardiovascular, hormonal, immunological and haematological systems.1 These changes mainly aim to provide favourable conditions for fetal development. The multiorgan changes induced by pregnancy also can affect the eye. Previous studies revealed that meibomian gland, cornea, conjunctiva, crystalline lens, refractive state, choroidal thickness (CT) can be affected in pregnant women.2–7 In most cases, the above changes are reversible and temporary. They are fully reversed in the months after delivery. However, sometimes they can be pathological and permanent, such as central serous chorioretinopathy, serous retinal detachment during pregnancy and vascular changes due to pre-eclampsia. Some studies speculated that these changes may be attributed to altered hormonal levels and altered ocular blood flow.8 9
The most significant systemic changes during pregnancy are haemodynamic state and hormone levels. Haemodynamic change is mainly to meet the demand for increased blood circulation. Plasma volume increases by 50% and reaches its peak at 32 weeks of gestation.10 Also, there is an increase in cardiac output, peripheral vasodilation and a fall in systemic vascular resistance.11 In addition, another significant change during pregnancy is hormone levels. Oestrogen, progesterone and renin–angiotensin levels increase markedly in pregnancy, which play an important role in regulating vascular endothelial cells, as well as the systemic and ocular vasculature.12
Previous research evaluated ocular vascular changes during pregnancy, which mainly focused on retinal and choroidal microcirculation.13 14 Microcirculation is a system consisting of arterioles, venules, capillaries and arteriovenous anastomosis. Normal microcirculation is the basis of retinal and choroidal function. In addition, studying the microcirculation of the eye helps to understand the vascular effects of systemic diseases. Optical coherence tomography angiography (OCTA) is a non-invasive technology that allows us to visualise the retinal and choroidal vasculature without the need for intravenous contrast agent and eye-drops. Some parameters can be safely obtained using OCTA, such as retinal superficial capillary plexus (SCP) vascular density (VD), retinal deep capillary plexus (DCP) VD, foveal avascular zone (FAZ) and choriocapillaris (CC) VD.15 Some studies have observed retinal and choroidal microvasculature features in pregnant women using OCTA. However, because of the limited sample size of the clinical trials, the outcomes were inconsistent. Given the discrepancy, we performed a detailed meta-analysis based on PICO (Population, Intervention, Comparator, Outcome) questions to analyse retinal and choroidal microvasculature features during pregnancy and provide deep understanding of microvasculature changes in pregnant women. The detailed PICO questions are shown in online supplemental material 1.
Supplemental material
Methods
Search strategy
This meta-analysis was performed in compliance with the Cochrane Collaboration recommendations and the results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed, Embase, Ovid, Web of Science and Cochrane Library were searched for published eligible studies from inception to November 2023. The search strategy was based on a combination of terms: (1) ”pregnancy” or ”pregnant” or ”gravid” or “gravids” or “gestation” or “conception”; (2)”retinal microvasculature” or “choroidal microvasculature” or “superficial capillary plexus” or “SCP” or “deep capillary plexus” or “DCP” or “foveal avascular zone” or “FAZ” or “choriocapillaris” or “CC” and (3) “optical coherence tomography angiography” or “OCTA” (online supplemental file 2).
Supplemental material
Study selection
In this meta-analysis, the inclusion criteria were as follows: (1) Studies comparing retinal microvasculature features or choroidal microvasculature features between pregnant women and non-pregnant women. (2) The parameters in the studies included SCP-VD, DCP-VD, FAZ and CC-VD. (3) The parameters were measured by OCTA. (4) Case–control studies involving human subjects. (5) Articles published in English were considered qualified.
In this meta-analysis, the exclusion criteria were as follows: (1) They were reviews, case reports, animal studies, conference abstracts, comments, posters or duplicate studies. (2) There are no sufficient data or data could not be extracted. (3) The included pregnant women and non-pregnant women were diagnosed with certain systemic diseases, such as hypertension, diabetes, pre-eclampsia, anaemia, etc.
Data extraction
Two authors independently extracted the data from the included articles. If opinions diverged, disagreements were resolved by consulting a third author and discussion. According to the Cochrane Handbook, the following data were extracted from the included studies: the first author of each study, year of publication, country, the study design, the number of enrolled participants, the mean age, the gestation weeks, OCTA device and parameter results.
Quality assessment
Quality assessment of the included studies was performed using the Newcastle-Ottawa Scale (NOS). The evaluation criteria include subject selection (4 points), subject comparability (2 points) and exposure assessment (3 points). The result of the quality assessment is a score range of 0–9 points. Studies with a total score <4 points were considered to be of low quality. The evidence quality of outcomes were evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method. In terms of risk of bias, inconsistency, indirectness, imprecision and publication bias, evidence quality was divided into ‘high quality’, ‘moderate quality’, ‘low quality’ and ‘very low quality’.
Statistical analysis
The Review Manager (RevMan) software (V.5.3.1) (Cochrane Collaboration, Oxford, UK) was used to analyse the extracted data. Continuous variable statistics were presented as the mean±SD, and mean differences (MDs) with its 95% CI were evaluated for pooled effect. Heterogeneity among studies was assessed using χ2 statistic test and I2 statistic test. I2>50% was considered to be statistically significant heterogeneity. A fixed-effect model was adopted if there was no significant heterogeneity among studies (I2<50%), otherwise a random-effect model was used. The publication bias was evaluated by funnel plots. A p<0.05 was considered statistically significant. According to the distribution-based methods, we considered the minimal clinically important difference (MCID) as a difference of 0.85% in SCP VD, 0.46% in DCP VD, 0.01 mm2 in FAZ, 0.96% in CC VD.16 17 When the differences between healthy pregnant women and non-pregnant controls were smaller than MCIDs, although p<0.05, we considered the difference as small and not clinically important.
Patient and public involvement
None.
Results
Search results
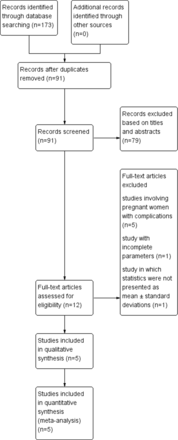
The detailed flow chart of retrieval and screening process is shown in figure 1. A total of 173 articles, including 91 studies potentially useful, were obtained from all databases after duplicates were removed. Two reviewers independently screened the titles and abstracts and excluded 79 articles. Then, 12 articles were assessed for qualified ones. These 12 records were screened for the abstract and full text. Among them, five studies focused on pregnant women with systemic complications, one study had incomplete parameters and one study in which statistics were not presented as mean±SD was also excluded. Ultimately, five studies18–22 were selected for inclusion. The five included articles were published from 2019 to 2023 and involved 219 eyes of pregnant women and 186 control eyes of non-pregnant women. All these studies were non-randomised comparative observational studies. The extracted general characteristics of included studies are shown in table 1.
Flow diagram of study selection and search results.
General characteristics of the included studies in the meta-analysis
Quality assessment
NOS quality assessment scores of the included studies were 6–8. They were considered to be of fair to good quality. The detailed quality assessment results of included studies are displayed in table 1.
We used the GRADE method to evaluate the quality of evidence for the primary outcomes: SCP VD, DCP VD, FAZ and CC VD. According to five degradation factors, namely risk of bias, inconsistency, indirectness, imprecision and publication bias, the results suggested that the evidence quality of SCP VD and CC VD was low and the evidence quality of DCP VD and FAZ was moderate. The detailed assessment results of evidence quality are shown in table 2.
Evaluation of GRADE evidence quality
Meta-analysis
The retinal SCP VD analysis in pregnant women and controls
Four studies with a total of 360 eyes (197 eyes in the pregnancy group and 163 eyes in the control group) reported the retinal SCP VD, but with significant heterogeneity across studies (χ2=20.54, p=0.0001, I2=85%). The difference between the pregnancy group and the control group was uncertain (MD 0.41; 95% CI −1.26 to 2.09; I2=85%; p=0.63). The results are shown in figure 2.
Forest plot for superficial capillary plexus vascular density (SCP VD), deep capillary plexus VD (DCP), foveal avascular zone (FAZ) and choriocapillaris (CC) VD between healthy pregnant women and non-pregnant controls.
The retinal DCP VD analysis in pregnant women and controls
Four studies with a total of 360 eyes (197 eyes in the pregnancy group and 163 eyes in the control group) reported the retinal DCP VD. There was minimal heterogeneity across studies (χ2=0.14, p=0.99, I2=0%). The retinal DCP VD was significantly higher in pregnant women than in controls (MD 1.53; 95% CI 0.62 to 2.44; I2=0%; p=0.0009). The results are shown in figure 2.
FAZ analysis in pregnant women and controls
Four studies with a total of 360 eyes (197 eyes in the pregnancy group and 163 eyes in the control group) reported the FAZ, with minimal heterogeneity across studies (χ2=3.50, p=0.32, I2=14%). The difference between the pregnancy group and the control group was uncertain (MD 0.01; 95% CI −0.01 to 0.03; I2=14%; p=0.18). The results are shown in figure 2.
CC VD analysis in pregnant women and controls
Three studies with a total of 194 eyes (82 eyes in the pregnancy group and 112 eyes in the control group) reported the CC VD. However, significant heterogeneity still existed (χ2=9.69, p=0.008, I2=79%). The difference between the pregnancy group and the control group was uncertain (MD 0.76; 95% CI −1.11 to 2.64; I2=79%; p=0.43). The results are shown in figure 2.
Discussion
In the present study, we compared the retinal and choroidal microvascular features in pregnant women and non-pregnant controls using meta-analysis. Some previous studies have observed and elucidated the difference, but the results were controversial.18–22 This study proved and expanded previous research to improve the quality of the evidence. As far as we know, this is the first meta-analysis study that reports the retinal and choroidal microvascular features detected by OCTA during pregnancy. This meta-analysis indicates that the DCP VD of the healthy pregnant women was significantly higher than that of the non-pregnant controls. However, uncertain difference was observed in retinal SCP VD, FAZ and CC VD between healthy pregnant women and non-pregnant controls.
In humans, pregnancy is accompanied by dramatic changes in hemodynamics, levels of various hormones and vasoactive metabolites. It has been suggested that haemodynamic changes during pregnancy occur through autonomic control mechanisms.11 Furthermore, the sympathetic nervous system plays a role in haemodynamic homeostasis during pregnancy. Previous studies have proposed that a fall in systemic vascular tone primarily triggered maternal haemodynamic adaptation to pregnancy. Cardiac output rises 40% during pregnancy responding to this decrease in systemic vascular resistance and the increase of blood volume.23 The peripheral arterial vasculature is maintained in a active vasodilation condition by continuous synthesis of endothelium-derived nitric oxide formed from L-arginine.12 24 25 As we know, pregnancy per se can lead to sympathetic activation despite a normal blood pressure. However, In contrast with the sympathetic activation, peripheral vascular resistance was found to be lower in normal pregnant women than that in non-pregnant women. Oestrogen may weaken the impact of sympathetic activation on vascular resistance through local mechanisms in humans.24
The regulation of the ocular circulation is complex. There are substantial differences between retinal circulation regulation and choroidal circulation regulation. Retinal blood flow is mainly determined by autoregulatory mechanisms and local factors while choroidal blood flow is mainly controlled by autonomic innervation. The flow through a vascular bed is determined by both perfusion pressure and vascular resistance. However, several studies have suggested that the influence of a moderate change in perfusion pressure on retinal blood flow is negligible. So it can be inferred that mechanisms in the retinal circulation that adapt vascular resistance to changes in perfusion pressure make retinal blood flow constant. Unfortunately, the detailed mechanisms are not yet fully understood. In contrast with retina, the choroidal circulation is mainly under neurogenic control and is not autoregulated. Some researches revealed that electrical stimulation of sympathetic and parasympathetic nerves had an effect on choroidal perfusion.24 26
This meta-analysis indicates that the DCP VD of the healthy pregnant women was significantly higher than that of the non-pregnant controls, but uncertain difference was observed in retinal SCP VD and FAZ between healthy pregnant women and non-pregnant controls. Retinal circulation is characterised by lower blood flow than that of choroid. Retinal circulation lacks autonomic innervation, which shows efficient autoregulation and is mainly affected by local factors. In addition, the influence of hormones and neurotransmitters on retinal vascular resistance is generally supposed to be negligible because of the blood–retinal barrier. Although pregnancy is associated with a decrease in vascular resistance and dramatic increase in blood volume, blood flow in retina is always in a constant state by autoregulation. In our meta-analysis, the difference in SCP VD and FAZ between healthy pregnant women and non-pregnant controls was uncertain. This result was consistent with the characteristics of retinal blood flow regulation. On the contrary, DCP VD of the pregnant women was significantly higher than in the non-pregnant controls, which meant higher DCP blood flow during pregnancy. Histological data from humans and other mammalian species have illustrated great variations in the density and laminar structure of the superficial and deep retinal capillary networks.27–29 Chandrasekera et al reported that capillaries composing the SCP were derived directly from precapillary arterioles and drained directly into postcapillary venules while no evidence was found where precapillary arterioles connected directly to capillaries composing the DCP. Furthermore, in the same plane as the SCP, there were major branches of the retinal artery and vein, while at the level of the DCP, segments of retinal arterioles and venules were not seen, and the DCP was characterised by numerous closed vascular loops. Not only that, available data supported a model in which major arterial inflow occurs at the level of the SCP while venous outflow preponderantly tracks through the DCP.30 In pregnancy, there is an increase in DCP perfusion density. The possible reason is that venous dilation secondary to increased blood volume and higher nitrous oxide levels predominantly occurs in DCP. The series and parallel organisation of peripapillary capillary plexuses may also make SCP and DCP different haemodynamics characteristics.31
The choroid is a complex structure consisting of vascular and stromal components, which are involved in physiological function and provides oxygen and nutrition for the outer layer of the retina.13 In the choroid, the flow per perfused volume is higher than any other human tissue, and choroidal blood supply provides 85% of total ocular blood flow.32 In addition, the ability of the choroidal vasculature to regulate its blood flow is far more restricted than that of retina because of the absence of glial cells, the considerably reduced pericyte ensheathment of the choroidal vasculature and the lack of intermediate filaments in choroidal pericytes.26 Therefore, the anatomical and functional characteristics of choroid may make it susceptible to the changes in haemodynamics during pregnancy. However, uncertain difference was found in CC VD between healthy pregnant women and non-pregnant controls. Jiang et al13 reported that the CT of the healthy pregnant women was significantly higher than that of the non-pregnant women. However, Su et al22 found that there was no significant difference between the pregnant and control groups in subfoveal CTs, Choroidal Vascularity Index or choriocapillaris flow deficits (CC FD). When calculating CC VD, both the vascular and interstitial components of the choroid are taken into consideration. Therefore, CC VD was believed to be more trustworthy and less affected by confounding factors such as intraocular pressure, axial length and systolic blood pressure than CT.22 What’s more, pregnancy-induced hormone changes may get involved. Oestrogens exert an influence on vasodilation while progesterone and renin–angiotensin increase the resistance of the ophthalmic artery and its branches. Hence, the mixed effects of hormones may lead to uncertain changes in the choroid, such as hyperperfusion, hypoperfusion of the choroid or no change at all.31
Our study has some limitations. First, the total sample size in the included studies was relatively small, which may make the quality of the evidence relatively low. Second, the pregnancy period of the included data was difficult to guarantee the consistency. At different pregnancy periods, changes in the retinal and choroidal microvasculature may be different. If there are enough data that focus on different pregnancy periods, further meta-analysis can be conducted. Third, except for pregnancy period, there still exists heterogeneity across the included studies. The potential causes of the heterogeneity may be study designs, eye condition (such as the dioptres of the eyes and the intraocular pressures), the type of OCTA devices used to obtain the metrics, algorithms used by the different OCTA device and different scan size. The heterogeneity of the included studies may threaten the validity and reliability of results. However, because of possible incomplete reporting of data, we cannot perform meta-regression to illuminate the source of heterogeneity.
In conclusion, our meta-analysis found that the DCP VD of healthy pregnant women was higher than that of non-pregnant controls. However, the SCP VD, FAZ and CC VD came to the opposite conclusion; uncertain difference was found between the healthy pregnant women and the non-pregnancy controls. Our meta-analysis revealed more convincing results by a larger sample size compared with the individual studies. In addition, our findings can help to get a deeper understanding of retinal and choroidal microvascular characteristics during pregnancy.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants but the ethical approval of this meta-analysis was exempted according to the Institutional Review Board of the second hospital of Hebei Medical University. This meta-analysis used statistically processed data and did not involve data of individual patient. In addition, all included articles were approved by the institutional review board of hospital or university where the clinical trials were conducted. This meta-analysis used statistically processed data which was extracted from the published articles and did not involve data of individual patient.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors PZ and QS designed the study. PZ, CW and YL searched the articles and contributed to data acquisition. PZ was responsible for processing data and writing original manuscript. QS and PZ revised the manuscript. All authors read and approved the final manuscript. QS is the guarantor.
Funding This study was supported by the Natural Science Foundation of Hebei Province (No. H2022206287) and S&T Program of Hebei (22377754D).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.