Article Text
Abstract
Introduction Current traveller health surveillance is ‘top-down’. Mobile-based surveillance could capture infection symptoms in real time. We aimed to evaluate the spectrum of illness in travellers using a mobile app-based system.
Methods This study (ClinicalTrials.gov NCT04672577) used an application called Infection Tracking in Travellers (ITIT) that records travel-related illness symptoms with associated geolocation and weather data. The free ITIT app is available in 14 languages. Participants were recruited globally from April 2022 to July 2023. Participants >18 years of age travelled internationally and provided electronic consent. Incentives included the provision of travel health information imported from the WHO website. Symptoms were recorded with daily pop-up questionnaires and symptom severity was assessed using a Likert scale. Two post-travel questionnaires were administered. Logistic mixed models examined factors relating to symptom presence, and a random forest model examined symptom impact.
Results 609 participants were recruited until July 2023. Participants had an average age of 37 years (18–79), and an average travel duration of 26 days (2–281). Most participants were travelling for leisure/tourism (401; 66%), followed by ‘visiting friends and relatives’ (99; 16%) and business travel (80; 13%). All continents were visited by at least one traveller. Of 470 registered trips, symptoms were reported on 163 trips (35%). Gastrointestinal symptoms were reported on 87 trips (19%) and respiratory symptoms on 81 trips (17%). The most important factors in predicting the presence of symptoms were duration of travel, travelling in winter and high humidity. Diarrhoea, headache and nausea were symptoms with most impact on daily activities. Post-travel questionnaires showed that 12% of surveyed participants experienced symptoms with several episodes of self-treatment. Two diagnoses were recorded: Lyme disease and amoebic dysentery.
Conclusion The digital tool ITIT successfully captures the spectrum of travel-related illness. This detailed epidemiology is crucial for outbreak detection and for the formulation of travel medicine guidelines.
Trial registration number NCT04672577.
- malaria
- epidemiology
- public health
Data availability statement
Data are available on reasonable request. Restrictions apply to the availability of the data that support the findings of this study and so are not publicly available. Some data can be made available from the authors on reasonable request and with permission of PS.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Provides real-time surveillance data on travel-related illnesses through a ‘bottom-up’ approach.
Links geolocation and environmental data with symptom reports for precise epidemiological profiling and illness cluster identification.
Uses a non-commercial digital tool for public health surveillance of travellers’ health.
To date, focuses mainly on European travellers which may influence the representativeness of the data.
The presence of missing data points could diminish the overall data quality.
Introduction
International travel is an integral part of life, whether for tourism, migration, business, or visiting friends and family, living in a different country. International mobility also exposes travellers to a range of health risks. Depending on the destination, traveller characteristics and purpose of travel, travel is associated with a broad spectrum of illnesses, including gastrointestinal complaints, respiratory infections and vectorborne diseases such as malaria and dengue.1 2 In addition, travellers can introduce pathogens to new regions and initiate disease outbreaks on return to their home countries particularly in vulnerable regions with conducive transmission conditions.2 3 Travellers’ mobility and exposure to infections in different global regions make them valuable sources of data on disease transmission patterns and key sentinels for monitoring and detecting potential outbreaks.4 Therefore, early detection and reporting of travel-related illnesses are crucial to implementing effective public health measures and safeguarding both travellers and the communities they interact with. In addition, recommendations for the protection of travellers’ health need to be evidence-based and up to date with respect to infectious disease epidemiology.
Historically, ‘top-down’ reporting has been the go-to method of tracking travel-related illnesses. These systems rely on healthcare professionals, laboratories and official health authorities to report mandatory infections or cases of interest regionally and nationally. However, there are several significant drawbacks to this approach. First, there is often a time lag in data reporting, as information must be logged, recorded and sent to relevant health agencies before it is available. Second, the data collected may lack crucial details that travellers themselves can provide and be inconsistent in reporting quality. Lastly, it relies on travellers attending medical facilities and seeking care, and such systems consequently do not capture less severe or asymptomatic cases, resulting in an incomplete picture of the actual disease burden.5 Surveillance networks that collate clinician-verified data on travellers’ illness such as EuroTravNet1 or GeoSentinel6 are limited by a lack of denominator data and also capture only a small portion of travel-related illness with a focus on severe illness. ‘Bottom-up’ symptom reporting by travellers themselves therefore offers a revolutionary solution to these challenges and an invaluable tool to supplement existing surveillance systems. There are several advantages of a real-time bottom-up reporting system. First, it ensures the timely detection of illness clusters, allowing for prompt investigation and intervention. This can facilitate rapid interventions, preventing localised outbreaks from spreading globally. Public health authorities can implement containment measures, quarantine protocols and vaccination campaigns promptly, curbing the progression of diseases. Second, travellers’ self-reports can provide valuable insights into environmental exposures, regional risk factors and potential disease hotspots, aiding in targeted preventive strategies to protect vulnerable populations. Lastly, the system fosters a sense of shared responsibility among travellers in safeguarding public health.
The widespread adoption of smartphones and digital platforms presents an unprecedented opportunity to implement a bottom-up, self-reported, illness tracking system. By encouraging travellers to report their symptoms and health conditions in real time through user-friendly mobile applications, a vast amount of data can be collected in real time, more accurately representing the true prevalence and distribution of travel-related illnesses. Research has shown that a majority of travellers are also willing to fill out symptom surveys and have their associated location tracked.7 However, with the advent of this quickly accessible data, it is more important than ever to consider the ethical implications and ensure privacy, and security for participants.8 Another issue in participatory studies is the retention and motivation of participants. We obtained travel health information from WHO in a format uploadable to the app as an incentive to take part in the study. Using the Infection Tracking in Travellers (ITIT) Travelhealth app, travellers report daily symptoms through a short, user-friendly questionnaire, and this information is then linked to location data as well as climate and air quality information. The app also collects demographic information and follows up with travellers after their trip to gain information on any persisting symptoms, self-treatments or confirmed medical diagnoses. More detailed information about the app can be seen in the pilot study, which looked at ease of use and feasibility of using the app, with promising results.9 This study evaluates data collected through the ITIT app from the first 609 recruited participants and examines the epidemiological patterns of reported symptoms by traveller demographics and location.
Methods
Patient and public involvement
The public was involved in this study as pilot participants, giving feedback for the ITIT app, suggesting improvements and modifications and demonstrating study feasibility.9 A feedback button on the app allows for participants to give input throughout their participation.10
Recruitment
Participants were recruited from 1 April 2022 to 15 July 2023 through a convenience sampling approach in travel clinics in Switzerland, Berlin, Amsterdam and partners of the ITIT global network, as well as through university-wide emails, conference promotions, public promotional material and word-of-mouth. The ITIT app is free of charge and available on the Apple App store and Google Play store, and information regarding the study, including a completely electronic informed consent form is found on the app. When participants download the app, they click through the informed consent, sign it electronically and then complete a preliminary demographic questionnaire. This questionnaire collects information about the traveller (>18 years old) and their trip, including the date and duration of their trip (minimum travel duration of 2 days). This information is then used to prompt pop-up reminders for the participants to complete the daily survey on each day of their trip. The daily survey collects information about the symptom type (gastrointestinal, respiratory, dermatological and general) and intensity of symptoms (6-point Likert scale: ‘none’, ‘mild’, ‘moderate’, ‘bad’, ‘very bad’ and ‘medical visit’) and the impact of these symptoms on the participant’s day on a 7-point Likert scale ranging from no impact on activities to hospitalisation. The daily survey can be filled out in less than a minute. Finally, after the trip is completed, participants are sent a follow-up questionnaire 7 and 28 days post-travel. This questionnaire retrieves information about symptoms that may have occurred after the trip and also about any diagnoses or medications used for self-treatment. As an incentive to take part in the project, the travellers are also provided with travel health information published by the WHO, freely available on the app. This information includes general travel-health information, specific vaccination information and disease outbreak news known as DONs (Daily Outbreak News) via API from the WHO and updated in real time.
Data storage and weather data
All the self-reported symptom and demographic information is linked to location and climate data and stored on secure servers in Zürich, Switzerland. The climate information is fed via the weather API from OpenWeatherMap and includes data on temperature, weather, humidity and air quality. These linked data were tied to the daily surveys and tagged with anonymised participant and trip IDs, as participants were able to take part in the study for multiple trips.
Statistical analysis
Demographic questionnaires were linked to the daily questionnaires using the trip ID column. Descriptive statistics were compiled based on the demographic information, including an analysis of average age, proportion of travellers with chronic diseases or smoking status and average trip duration. Using the linked location data, a map of daily surveys was created showing the presence and intensity of symptoms.
The absolute number of all reported symptoms was calculated both individually and in symptom groups (gastrointestinal, respiratory, dermatological and general) and then stratified by travel region and sex. The incidence rate of these reported symptoms was calculated by dividing the number of reported symptoms by the total number of completed surveys and then multiplying by 1000 to obtain the rate per 1000 surveys. This information was visualised in a heat map table.
Logistic mixed models were used to analyse participants’ daily surveys, taking into account the clustering of data by individual trips. These models assessed the influence of various factors on the likelihood of symptom expression, both overall and within four symptom subcategories.11 Univariate analysis was conducted first, followed by multivariate analysis based on the optimal model. The optimal model was determined by a combination of ‘order’ and ‘backward’ elimination, using the Akaike information criterion as the selection criterion. In the ‘order’ method, the terms are ordered according to their contribution to the model to ensure that the model converges before performing ‘backward elimination’.
Due to the large amount of missing survey data, multivariate imputation by chained equations (MICE) with 15 imputations was applied to the optimal models using linear mixed models for numerical data, two-stage logistic models for binary data and replication of the most likely value within a class for factors with more than two stages. These methods were chosen to account for the clustering of participants within their respective trip.
Several classification models were evaluated to predict the impact of symptoms on daily activities, including random forest, penalised logistic regression, XGBoost, Classification and Regression Trees (CART) decision tree and k-nearest neighbours. The models were carefully evaluated and tuned for optimal performance. The random forest model was selected as the best-performing model based on area under the curve (AUC) score.
A significance level of 0.05 was used for all statistical tests. All analyses and data processing were done using the statistical software R, V.4.2.3.
Results
In total, 609 travellers participated in the study. Of these, 401 (66%) were tourists, and 99 (16%) were visiting friends and relatives (VFR). The mean age was 37 years old, and 337 (55%) were female. A total of 501 (82%) of participants had never smoked, and only 58 (9.5%) had any comorbidities. The mean travel duration was 26 days (2–281), and the most common travel destination was Europe with 233 travellers (38%), followed by Asia with 145 (24%), the Americas with 115 (24%), Africa with 103 (17%) and Oceania with 11 (1.8%). Overall, 66% (n=404) of travellers who downloaded the app and filled out the demographic survey also filled out at least one daily survey. The response rate for these ‘active travellers’ was 46% (table 1).
Sociodemographic characteristics of ITIT participants (n=609)
Overall, there were 2905 daily symptom surveys with associated location data filled out by participants. Figure 1 shows the distribution of all the daily questionnaires, as well as if a symptom was reported, and if so, which symptom category it belonged to, and the symptom intensity. Almost the full range of symptom intensities and categories was seen with four surveys reporting symptoms prompting medical attention (see travellers’ details in online supplemental appendix 1. Some initial symptom clusters can be visually identified, including groups of symptoms around southeast Asia and central America, as well as southern Europe.
Supplemental material
Map of daily surveys with available Global Positioning System (GPS) location completed by ITIT participants, including symptom category and intensity (n=2905). The delimitation of continents is based on the Natural Earth Data V.4.1.0 (March 2018). Points located in international waters are associated with the nearest continent. ITIT, Infection Tracking in Travellers.
In total, there were 3739 surveys filled, when including surveys with no associated location data; of these, 512 reported some symptoms (14%). On evaluation of the symptom types reported, stratified by region of travel and sex, gastrointestinal symptoms are most frequently reported, with an incidence rate of 66.33 per 1000 completed surveys, and dermatological symptoms the least, at 25.41 per 1000 completed surveys. In addition, when looking at individual symptoms, diarrhoea is most often reported with 52.69 reports per 1000 surveys. In travellers visiting Asia, this rate increases to 90.46 per 1000 completed surveys. Women reported overall more symptoms than male participants (IR of 154 vs 115 per 1000) and reported more symptoms in all categories. Respiratory symptoms, mainly cough and a runny nose, were reported most frequently in Europe and were overall the second-most reported group of symptoms. No participants reported other body aches, and only 10 (0.03%) surveys reported swollen joints (table 2).
Absolute number and incidence rate of symptoms reported by travellers using the ITIT app, stratified by sex and location of travel (n=3739)
Of the 470 recorded active trips, travellers reported experiencing symptoms on at least 1 day during their travels on 163 trips, representing 35% of the total recorded active trips. The breakdown of symptoms reported is as follows: 87 (19%) trips reported at least one gastrointestinal symptom; 81 (17%) reported at least one respiratory symptom, 35 trips (7.4%) reported at least one dermatological symptom and 77 trips (16%) reported at least one general symptom. A total of 74 post-travel surveys were completed from 72 distinct travellers. Of these, 9 (12%) of the surveys reported travellers experiencing symptoms since their return. Furthermore, 24 (32%) of surveys reported self-treatment. These self-treatments included over-the-counter medications such as loperamide and paracetamol, antibiotics such as streptomycin, and other treatments including vitamins, mosquito bite balms and natural oils. Among those travellers reporting symptoms post travel, 2 (22%) sought medical attention and the same percentage received a medical diagnosis. One participant travelling to Italy and Australia reported a coinfection with Lyme disease and amoebic dysentery. One survey reported a diagnosis (common cold) without having any symptoms or consultation. No traveller reported hospitalisation.
When examining which factors influence the presence of reported symptoms using logistic mixed modelling, univariate analysis showed that duration of travel, age, location of travel to Asia, business travel, humidity and travelling in winter were significant at the 5% level. The optimised multivariate model using complete case analysis, however, only kept duration of travel, humidity, wind speed and season at destination, and of these, only duration of travel and winter travel is significant (OR 3.10, p<0.001 and OR 2.79, p 0.001, respectively). When looking at the MICE multivariate model, the same explanatory variables are kept in the model as the previously discussed mode, but in this case only duration of travel (OR 1.26, p=0.043) and humidity (OR: 1.76, p<0.001) were significant (see table 3).
Univariate and multivariate analyses of variables influencing symptom expression using complete case analysis and imputed full sample analysis
When examining symptom categories separately, the multivariate models using MICE showed different factors as being associated with symptom presence. Duration of travel, higher humidity and atmospheric ammonia (NH3 µg/m³) were associated with gastrointestinal symptom presence, whereas for respiratory symptoms and general symptoms, no factor was significantly associated with symptom presence in the imputed model. Duration of travel, higher temperatures and travelling in summer versus autumn were associated with higher incidence of dermatological symptoms (online supplemental appendices 2–5).
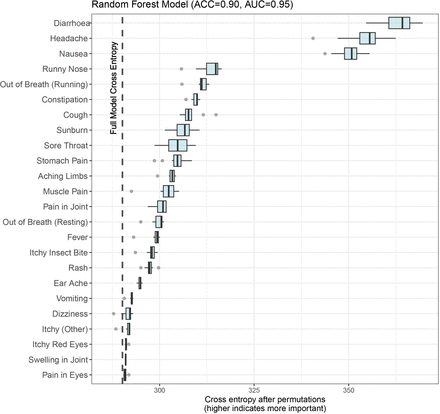
The random forest model, which predicts the impact of symptoms on daily activities with an accuracy (ACC) of 90% and an AUC of 0.95, indicates that diarrhoea, headache and nausea are the three most important symptoms for predicting the impact on a participant’s daily activities. These symptoms have an average cross entropy of 362.9, 354.5 and 350.3, respectively, representing a raise of 72.7, 64.3 and 60.1 from the full model cross entropy of 290.2. Other symptoms such as having a runny nose and being out of breath also have an impact, but to a lesser extent (figure 2).
Impact of symptoms on daily activities disturbances as measured by mean cross entropy raise after 10 permutations using a random forest model. The vertical line in the figure represents the cross entropy of the full model. Each row displays the new cross entropy of the model when the variable of interest is removed, shown as a boxplot with the mean cross entropy after 10 permutations. The larger the increase in cross entropy when the variable is removed, the more important that variable is to the model. ACC, accuracy; AUC, area under the curve.
Discussion
The ITIT project is a non-commercial, public health endeavour that enables travellers to provide ‘bottom-up’ travel-related, illness surveillance data in real time. In the first year of recruitment, over 600 travellers filled out over 3700 daily symptom surveys, travelling to every continent and displaying a wide range of symptom types and intensities. This study confirmed the feasibility of using ITIT for larger numbers of participants, reaffirming the conclusions of the pilot ITIT study.9 Travel across any international border qualified for participation and allowed for the surveillance of travellers’ health in Europe, a continent with the largest numbers of visitors worldwide but an area, which is often not on the surveillance radar. In addition, the epidemiological profile of travellers’ illness and initial hotspots of symptoms could be seen using the linked demographic and location information. A milestone with the ITIT app is the incentive for users to have access to information published by WHO on malaria risk and yellow fever/other vaccination requirements at the destination and also access via API to the WHO publication ‘DONs’.
With regard to possible participation bias, the target population for the ITIT project is all travellers who cross an international border and travel for 2 days or longer. Travellers do not form a homogenous group but rather encompass many types of travellers who are categorised by their purpose of travel—these include tourists, VFR, migrants, business travellers, visitors to mass events/others. This paper includes all these traveller types with tourists (66%), VFR (16%) and business travellers (13%) and a small number of mass gathering visitors. The proportions of these traveller types within the ITIT cohort correspond with other papers on travel-related illness1 12 (tourists 51%, VFR 14%, business 11%) and5 (tourists 63%, VFR 16.3% and business 14%). To avoid sex bias, this study evaluates data on approximately equal numbers of men and women, a wide range of ages and there are also short-term and long-term travellers. In ITIT, we aimed to include short-haul travel including travel to bordering countries in Europe. This is important as travel anywhere can be associated with infection dissemination. Our travellers were recruited mainly from travel clinics that see all the types of travellers listed above so our participants do reflect the travelling public in general. One possible bias may be that travellers who were more health conscious and willing to take part in citizen science were included in the dataset. The response rate of 46% for active travellers in this study was lower compared with a similar app-based travel health study (table 1). However, the number of participants and the total number of responses were significantly higher. In addition, the recruitment process was paperless and allowed for more flexibility and a broader range of recruitment with both passive (the travellers download the app themselves outside medical centres) and active (through travel medicine professionals) recruitment methods.12 We also sought to increase the participation of travellers attending mass gathering events such as the pilgrims to the Hajj in Saudi Arabia and visitors to sporting events such as the Winter Olympics in Beijing.
The full range of symptoms surveyed was reported, except for ‘other body aches’, which were not reported by any participant. Symptoms were reported by 35% of travellers, which is higher than previously reported estimates, with a study showing 15% of travellers to developing countries becoming ill.13 This is expected, as less severe symptoms will be caught by bottom-up, traveller-reported methods than most other studies which receive data from ‘top-down’ official health systems. A majority of gastrointestinal and respiratory symptoms was also seen as expected,14 with gastrointestinal issues being most common in travellers to Asia, where the risk of foodborne pathogens can be high. More participants would be needed to more clearly differentiate epidemiological patterns of symptoms by region, as Oceania did not have many travellers. Differences in illness symptoms for male and female travellers were also seen and have been reported in previous analyses of travel infection data.15 Some differences, such as the higher proportion of diarrhoea in females support previous literature16; however, the higher proportion of fever in women contrast with what has previously been observed, with males usually reporting more febrile illnesses.15 However, this difference may also be partially accounted for by differences in self-reporting habits between the sexes, although more research is needed here.
Multivariate modelling showed that the most important variables when looking at risk of symptoms overall are duration of travel, and either humidity or travelling during winter, with all three variables being associated with an increased risk of symptom presence. Humidity, atmospheric pressure and air pollutants were found to have a significant impact on some symptoms (online supplemental appendices 2–5) and larger numbers of travellers are needed to further elucidate these associations. Increased duration of travel increases the probability of symptom reporting.17 Winter travel, including winter travel in Europe, can be associated with increased respiratory illness due to cold temperatures and influenza seasons, and humidity was observed to be associated with increased respiratory illness prevalence.18 For travel consultations, this could mean that different illnesses and preventative measures should be emphasised depending on the season at the destination. Consistent with previous studies and observed in our results, older travellers exhibit fewer symptoms, likely due to their better adherence to travel health recommendations and prevention strategies.19 The impact of symptoms on the travellers’ day overall, using self-reported impact ratings showed that diarrhoea, headache and nausea were the three most important symptoms. This should guide recommendations for the most likely self-treatments needed during travel suggesting that medications such as paracetamol to treat headaches, loperamide for diarrhoea, and domperidone for nausea could be recommended in pretravel consultations.
Our study had some limitations; the recruitment for the study was mainly done through the EuroTravNet partners, which led to a majority of European travellers being recruited and destinations favoured by Europeans being over-represented. As a result, the incidence rate for less frequently visited destinations, such as Oceania, may be underestimated. Missing data points could potentially have decreased the quality of the data. This issue can also be observed in the analysis of under-represented symptom groups in our study, such as dermatological and general symptoms, where the estimation could be impacted. The intensive nature of the study selected for travellers who were perhaps more careful about their health or more likely to report symptoms. Ongoing recruitment will focus on recruiting larger numbers and a broader range of travellers and the creation of large datasets with possible artificial intelligence applications. The updated app will monitor persisting illness post-travel. The ITIT project has some major advantages compared with other travel health apps. These include having the WHO publications uploaded to the app, recruiting at many global locations outside Europe—recently extended to South Africa, Malaysia and Japan. Another advantage is the fact that the app is available in 14 languages and will be available for all categories of travellers independently of travel clinics. Compared with traditional surveillance systems, we suggest that ITIT captures a more accurate, granular picture of symptoms experienced by the traveller, with a future potential for outbreak detection due to the real-time and location-associated nature of the data when large numbers of travellers use the app.
Digital innovations in the health field, and travel health specifically, have already shown promise in the COVID-19 pandemic, whether through passive wearable technologies, or self-reported test results and symptoms.20–22 In an analogous manner, ITIT, using self-reported symptom surveillance in travellers has the potential to innovate the field of travel medicine, and supplement existing disease surveillance methods, giving real-time outbreak detection data, far before they would be registered by traditional means.
Conclusion
In conclusion, this era of global travel necessitates an evolution in the way travellers prepare for their trip and how we monitor and report travel-related illnesses and identify clusters of infections and possible alerts. Travellers can play an invaluable role as sentinels for outbreak detection and disease surveillance if large numbers are contributing data to a centralised system. By embracing real time, bottom-up symptom reporting, we can support existing programmes and improve global health surveillance.
Data availability statement
Data are available on reasonable request. Restrictions apply to the availability of the data that support the findings of this study and so are not publicly available. Some data can be made available from the authors on reasonable request and with permission of PS.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Swiss Ethics Committee (BASEC number 2020-02292). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We gratefully acknowledge Dr Mika Kawano and Dr Ninglan Wang, WHO, for their technical support to the ITIT project.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
TL and NH are joint first authors.
X @HedrichNadja, @PatSchlagenhauf
Correction notice This article has been corrected since it was published. The surname of author, Patricia Schlagenhauf, was misspelled.
Collaborators The ITIT Global Network: Ulf Blanke: Antavi GmbH, Krähbühlstrasse 58, 8044, Zürich, Switzerland. Gilles Eperon: Division of Tropical and Humanitarian Medicine, Geneva University Hospitals, Rue Gabrielle-Perret-Gentil 6, 1211, Geneva, Switzerland. Philippe Gautret : IHU - Méditerranée Infection, 19-21 Boulevard Jean Moulin, 13005 Marseille. Albie de Frey: University of the Witwatersrand; Travel Doctor Corporate, Johannesburg. Esther Kuenzli: Swiss Tropical and Public Health Institute, Socinstrasse 55, 4051, Basel, Switzerland. Andreas Lindner: Charité-Universitätsmedizin Berlin, Charité Center for Global Health, Institute of International Health, 13353 Berlin, Germany. Frank Mockenhaupt: Charité-Universitätsmedizin Berlin, Charité Center for Global Health, Institute of International Health, 13353 Berlin, Germany. Corneliu Popescu: Dr.Victor Babes Clinical Hospital of Infectious and Tropical Diseases, Bucharest, Romania. Jenny L. Schnyder: Center for Tropical Medicine and Travel Medicine, Department of Infectious Diseases, Amsterdam UMC, location University of Amsterdam, Amsterdam, Netherlands. Hanna K. de Jong: Center for Tropical Medicine and Travel Medicine, Department of Infectious Diseases, Amsterdam UMC, location University of Amsterdam, Amsterdam, Netherlands. Mohammed Dauda: Goni, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, 16100 Kota Bharu, Kelantan, Malaysia. Hiroshi Nishiura: Kyoto University School of Public Health, Yoshidakonoecho, Sakyoku, Kyoto City 6068501, Japan. Jaffar A. Al-Tawfiq: Specialty Internal Medicine and Quality Department, Johns Hopkins Aramco Healthcare, Dhahran, Saudi Arabia. Salim Parker: Division of Infectious Diseases and HIV Medicine, University of Cape Town, Cape Town, South Africa. Carsten Schade Larsen: Dept. of Infectious Diseases Q, Aarhus University Hospital, Skejby, 8200 Aarhus N, Denmark. Effy Vayena: Health Ethics and Policy Lab, Department of Health Sciences and Technology, ETH, Zürich, Switzerland.
Contributors TL: conceptualisation, methodology, investigation, data curation, formal analysis, visualisation, writing–original draft. NH: conceptualisation, methodology, investigation, data curation, formal analysis, writing–original draft. MPG: investigation, writing–review and editing. JB: investigation, writing–review and editing. PS: guarantor, project initiation and grant writing, funding acquisition, conceptualisation, methodology, data curation, supervision, validation, investigation, writing–original draft, review and editing.
Funding This study was funded by the Swiss National Science Foundation, Switzerland (grant number 320030_192653).
Disclaimer The funder played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Map disclaimer The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Author note The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.