Article Text
Abstract
Introduction Cancer-related fatigue is common in patients with advanced lung cancer. It not only interferes with patients’ health-related quality of life, but also increases the caregiving burden of their caregivers. Acceptance and commitment therapy is emerging as a novel way to advocate accepting negative experiences and taking effective actions based on their own values to help patients commit meaningful actions in the course of cancer diseases. This trial aims to test the feasibility, acceptability and preliminary effects of acceptance and commitment therapy for fatigue interference in patients with advanced lung cancer and the caregiver burden.
Method and analysis A two-arm, assessor-blind pilot randomised controlled trial will be conducted. A total of 40 advanced lung cancer patient–caregiver dyads, who live in rural areas, will be recruited from a university-affiliated hospital in central China. The participants will be randomised to receive an online six-session acceptance and commitment therapy (i.e. involving metaphors, experiential exercises and mindfulness exercises facilitated by virtual reality technology) plus health education (intervention group, n=20) or health education (control group, n=20). Outcomes will be measured at baseline and 1 week postintervention. The primary outcomes are study feasibility (i.e. eligibility rate, recruitment rate, attrition rate and adherence rate), fatigue interference and caregiver burden. The secondary outcomes are health-related quality of life, meaning in life, psychological flexibility and mindful attention. Semistructured interviews will be conducted to explore the feasibility and experiences of the intervention in a subsample of 10 participants from the intervention group.
Ethics and dissemination This study has been approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (CREC Ref. No. 2023.030) and the Medical Ethics Committee of Xiangya Hospital Central South University (No. 202305336). The findings will be disseminated in peer-reviewed journals and through local or international conference presentations.
Trial registration number NCT05885984.
- Fatigue
- Adult oncology
- Adult palliative care
- Randomized Controlled Trial
- Nursing Care
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The intervention was developed underpinned by the acceptance and commitment therapy model.
Eligible participants will be randomly assigned in a 1:1 ratio to either the control group or the intervention group to realise a balanced group allocation.
Both quantitative and qualitative analyses will be applied to evaluate the feasibility of the study.
This is a single-site study; therefore, findings may be dependent on local context.
Due to the nature of the intervention, blinding of participants and the interventionist will be impractical.
Introduction
Lung cancer has received considerable attention because of its leading incidence and mortality.1 Many patients with lung cancer are diagnosed in advanced stages. An advanced cancer diagnosis is often associated with multiple distress, which can be arisen from various factors, including physical symptoms disturbance, side effects of treatments, uncertainty about the future, changes in lifestyle and financial burdens, persisting for years after the completion of treatment.2 Cancer-related fatigue (CRF) is the most common and distressing symptom in patients with advanced lung cancer.3 4 The lasting impact of CRF, which is called fatigue interference,5 refers to the degree of CRF-associated interference with different aspects of life. CRF substantially interferes with not only patients’ abilities to participate in daily activities,6 but also changes in the psychological health7 and severely worsen their health-related quality of life (HRQOL).8
Fatigue interference and patients’ psychological distress may also influence their family members, which increases the difficulties of caring.9 Caregiver burden is a multidimensional concept related to the physical symptoms, psychological distress, impaired social relationships, spiritual distress and financial strain that arise from caregivers.10 11 In the context of advanced lung cancer, as psychological distress and associated fatigue interference affect patients’ functioning, family caregivers of lung cancer patients are required to spend longer time performing care duties for patients while neglecting their own vital activities.12 Coping with demanding role changes and increased caregiving burden can significantly disrupt daily routines, impact caregivers’ mental and physical health, thus influencing their sense of meaning in life.13 Family members have a key role in providing advanced lung cancer patients with informational, instrumental and emotional support, which is crucial to patients’ adaptation to the advanced illness and living a meaningful cancer life.14 Alleviating caregiver burden should thus be a critical priority given its impact on carers’ and care-recipients’ emotional, social, physical and spiritual functioning. Thus, it is highlighted that oncological treatment needs to be complemented by efficient psycho-oncological support for patients with advanced lung cancer and their caregivers.
Evidence-based interventions for improving both patient fatigue interference and caregiver burden in patients with advanced cancer is lacking. Psychoeducation,15 couple-based intervention,16 mindfulness-based interventions17 and online support programmes18 were investigated in cancer population, however, the evidence of beneficial effects was inconclusive due to the methodological heterogeneity (i.e. involving cancer patients at early stages, diversity of intervention mode and control and reporting bias). Importantly, these studies did not have a symptom or distress criteria for study entry. Thus, alternative interventions targeting at symptom interference and caregiver burden with rigorous design are warranted.
Acceptance and commitment therapy (ACT) is designed to maximise psychological flexibility in navigating life’s challenges and may reduce maladaptive coping while facilitating adaptive management of distress.19 ACT is a mindfulness-based behavioural therapy that combines a mix of metaphor and mindfulness techniques, as well as a variety of experiential exercises and value-guided behavioural interventions.20 21 Different from other psychological interventions, ACT does not focus on changing unhelpful thoughts and feelings but emphasises acceptance while living mindfully according to one’s values.22
Research has indicated the effects of ACT on improving distress and quality of life in cancer patients.23–26 A previous systematic review of ACT completed by our research team has also shown its promising effects on improving fatigue interference and HRQOL in patients with advanced lung cancer.27 Besides, a systematic review and meta-analysis involving 24 studies indicate the effects of ACT on psychological distress and quality of life in family caregiver population.28 However, only 12 studies were randomised controlled trials (RCTs), and only four studies focused on family members with cancer or in palliative care. Among these four studies, only Mosher et al 29 included both patients with advanced lung cancer and caregivers. Although the feasibility and acceptability of telephone-based ACT intervention were suggested, patients with moderate symptoms persistently bothered and caregivers with subclinical distress were recruited, which may limit the preliminary effects of ACT. Evidence on the ACT-based interventions that address distressing symptoms for patients and the caregiving burden for caregivers are still lacking.
It is noted that discharged patients with advanced lung cancer and caregivers have experienced extensive unmet supportive care needs during the COVID-19 pandemic. Especially for patients living in remote and rural areas in China, geographical constraints severely affect the HRQOL of patients and caregivers due to the lack of timely psychological support.30 The implementation of ACT intervention also encountered challenges in the COVID-19 situation from our research team, such as face-to-face conduct mode.31 Alternatively, increasingly digital-based ACT interventions have been developed and shown considerable effects for different populations.32 33 Digital interventions do not only have the advantage of cost-efficiency and high accessibility but could, therefore, particularly benefit those currently underserved.34 Studies also indicated that online-based ACT (e.g. videoconferencing-based sessions) not only provide patients at home with continuous psychological support, especially those in rural areas but may also have practical application prospects for those who are already financially strained.35 Moreover, some digital-based techniques, that is, virtual reality (VR),36–38 are increasingly applied to alleviate psychological distress in diverse populations. Immersive VR benefits isolating users visually and acoustically from a distress-inducing clinical environment and helps users focus on relaxing stimuli through a head-mounted display.39 However, few studies have combined the technology with ACT and applied it in patients with advanced cancer patients and their caregivers. Notwithstanding these limitations, this study may help provide initial evidence of an Online-based ACT interventions to address the unmet needs of advanced lung cancer patients–caregiver dyads.
Our research foundation
Some preliminary work on ACT studies in patients with advanced cancer has been done by our research team. The principal investigator completed a project on ACT-based intervention in patients with advanced lung cancer in China. An ACT-based intervention manual was developed and modified based on the research findings of our systematic review27 and qualitative study.40 Results of a rigorous full-scale RCT suggested that ACT can effectively improve fatigue interference and HRQoL in patients with advanced lung cancer in China.41 During the research period, most of the patients were from rural areas, they showed few challenges in attending the videoconferencing-based ACT sessions via their smartphones. In addition, their families showed great interests in attending the ACT interventions and expressed their unmet needs on their caregiving burden.
Theoretical framework
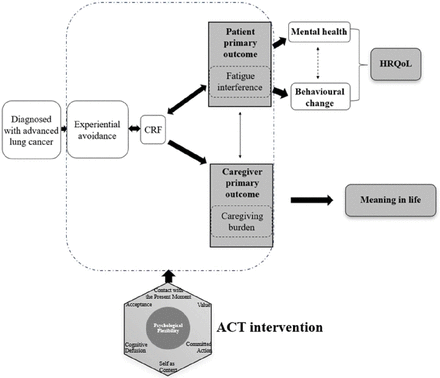
Being diagnosed with advanced lung cancer, symptom burden and side effects due to cancer treatment are often considerable.42 Most patients with advanced lung cancer patients inevitably experience varying degrees of CRF.43 Experiential avoidance due to CRF may reduce the tolerance to psychological reactions. Over time, individuals may resort to strategies to avoid or reduce CRF to limit their daily activities, and their physical functions are decreased, psychological distress is increased and the overall HRQOL is thus reduced. Therefore, putting psychological flexibility into the context of the illness trajectory of advanced cancer, hypothetically (see figure 1), by receiving the ACT-based intervention, patients with advanced lung cancer become more willing to accept the CRF experience and fatigue interference. They are able to understand that thoughts about distressing CRF experiences are merely thoughts rather than facts and should be taken lightly, and fatigue interference could be decreased. On the other hand, family caregivers can flexibly cope with challenges, difficulties and strains as a result of their caregiving responsibilities. Rather than immersing in emotional distress related to caregiving burden, they are able to reclarify their life directions and take value-oriented actions. Furthermore, the decrease in fatigue interference may further relieve stress of their caregivers, decrease the impacts of the caregiving burden on their daily lives and therefore improve caregivers’ meaning of life and subsequently enable patients to live a better quality of life.
Hypothetical theoretical framework of the study. ACT, acceptance and commitment therapy; CRF, cancer-related fatigue; HRQoL, health-related quality of life.
Based on our previous research basis, the ACT intervention in patients with advanced lung cancer31 will be modified to target fatigue interference in patients with advanced lung cancer and their caregivers with caregiving burden in China. With the rapid economic development in China, wired and Wi-Fi networks have become widespread in rural areas in China, and the utilisation of mobile phones has gradually become common among the elderly in rural China.44 This provides convenience for healthcare professionals to aid their mental well-being using digital platforms.
Thus, a pilot RCT will evaluate the feasibility and acceptability of an online-based dyadic ACT intervention involving 360 degree VR technology supported mindfulness for patients with advanced lung cancer and their caregivers in rural areas.
Methods and analysis
Aims and objectives
This study aims to test the feasibility, acceptability and preliminary effects of the online six-session ACT intervention in improving fatigue interference for patients with advanced lung cancer and the caregiving burden for caregivers in rural areas in China.
Specific objectives are as follows:
To investigate the feasibility and acceptability of the online six-week ACT in patients with advanced lung cancer and caregivers in rural areas in China.
To investigate the preliminary effects of the online six-week ACT in patients with advanced lung cancer and caregivers in rural areas in China.
To explore how patients and caregivers perceive the ACT experience during the intervention process.
Design
A two-arm, assessor-blind pilot RCT will be implemented to investigate the feasibility, acceptability and preliminary effects of ACT for advanced lung cancer patient–caregiver dyads.
Study setting and participants
Participants will be recruited by using convenience sampling from the respiratory department in a university-affiliated hospital in central China.
Eligible patients are (1) aged 18 or over; (2) diagnosed with stage III or stage IV lung cancer; (3) lived in rural areas; (4) experienced unexplained fatigue syndrome: the score of 3 or more on the Fatigue Symptom Inventory (FSI); (5) able to provide informed consent and effectively collect data; (6) have a consenting primary family caregiver and (7) have working phone service. Patients who are (1) diagnosed with cognitive dysfunction or other mental illnesses; (2) in critical condition with any major concurrent medical disease and (3) who are receiving or have just completed other cancer-related intervention programmes within the last 6 months will be excluded.
Caregiver eligibility criteria are as follows: (1) aged 18 or over; (2) primary family caregiver who either lives with the patient or has visited them at least twice a week for the past month; (3) reported caregiving burden (score of 12 or more on the Zarit Burden Inventory;45 (4) have working phone service and (5) lived in rural areas. Caregivers who have (1) cognitive impairments or (2) severe diseases will be excluded.
The sample size is calculated using G*Power. A stepped rule of thumb for pilot studies based on the anticipated effect size of a future main trial46 was adopted to guide the sample size planning for the present pilot study. Allowing for a moderate effect size of 0.5 with a study power of 90% at a significance level of 5% (two-tailed) in the future main trial, a sample of at least 30 participants (15 participants per arm) should be considered adequate. Considering a reported attrition rate was 25% at postintervention in our previous pilot study examining an ACT intervention by both face-to-face and videoconferencing platform in patients with advanced lung cancer31. Therefore, a total of 40 dyads, including patients with advanced lung cancer and caregivers, should be recruited for this pilot study (20 per group).
Recruitment
Figure 2 shows the procedural flow of this study. Patients will be identified for potential inclusion through a review of their medical records. A clinical nurse will then approach these individuals to assess their eligibility. A list of interested, eligible patients will be documented to facilitate researcher contact. Interested patients identify a family caregiver and are screened for eligibility. With the patient’s permission, up to three family caregivers are consecutively screened for eligibility. If none of the caregivers is eligible and consents to participate, then the patient is ineligible for study participation. Potential dyads will receive information about the study and provide written informed consent via electronic signature by WeChat (A popular Chinese social media app) within 48 hours. After the participants approach the eligible assessment and provide written informed consent, an online survey link through Sojump (a popular service provider in China engaged in online questionnaires, examinations and voting platforms) will be sent to them via WeChat to complete the baseline assessment.
Flow diagram of this study.
Randomisation and blinding
The randomisation for this study will be performed by a research assistant who is independent of the recruitment, enrolment and treatment processes. Participants will be randomly assigned to the intervention or control group at a 1:1 ratio. This will be achieved using a permuted block design (block sizes: 4, 6), implemented through a computer-generated random sampling procedure from Sealed Envelope (https://www.sealedenvelope.com/). This method will ensure a balanced allocation of participants between the two study arms. Allocation of participants will be informed to interventionists through email, whereas participants will be informed by an opaque and sealed envelope with a generated sequence by the research assistant. The allocation list will also be concealed in an encrypted file on a password-protected computer.
Given the psychological nature of the intervention, blinding the interventionist and participants will not be possible. However, the assessors who collect data and perform analyses will be blinded to the group allocations. To prevent contamination, participants in both groups will be instructed to avoid discussing the intervention content with others and disclosing their group allocation to the assessors.
Interventions
Intervention group: ACT plus health education
The ACT intervention, which is modified based on our previous ACT intervention manual in patients with advanced lung cancer,31 consists of 6 weekly individual sessions of 60–90 min each, in addition to health education. Modifications included increasing the number of sessions provided from four to six sessions due to increased caregiver parts. The original last two sessions involving value clarification and goal setting for patients only were changed to joint sessions including both patients and caregivers.
Participants randomised to the online ACT group will complete the 6 weekly online sessions via a videoconferencing platform (i.e. Tencent conferencing, a popular social media app in China, which has equal functions with Zoom). The intervention consists of two sessions for patients only, two sessions for caregivers only and two sessions for dyads (table 1). The participants will receive a reminder of appointments via WeChat in advance. Strategies consisted of homework assignments, relevant ACT metaphors and experiential exercises, 360 degree VR technology-supported mindfulness exercises are included to cultivate the process. Metaphors47 with experiential exercise are the unique strategy in ACT different from other psychological interventions. Mindfulness constitutes one of the most well-known and empirically supported sets of exercises employed in therapies that emphasise contact with experience over changing thoughts. During the intervention, the patients will experience the sense of immersion with a first-generation headset by inserting a smartphone with mindfulness animations into the VR headset. A trained research assistant will provide the dyads with standard instructions on how to use the VR equipment to practice mindfulness at home. The VR headset will be guided to be placed and adjusted on the participant’s head to ensure comfort and secure fit and familiarise themselves with the headset prior to actual intervention. Each subprocess of ACT can be targeted with relatively specific techniques. A patient booklet and a caregiver booklet will be distributed to the patients and caregivers before the first session, and a structured practice log will be used to record their homework completion. The intervention programme will be finally validated by the expert panel.
Intervention outline
Control group: health education
Participants randomised to the control group will receive 6 weekly health education (20–30 min each) via Tencent conference software. The education consists of two sessions for patients only, two sessions for caregivers only and two sessions for dyads. The topics mainly include treatments and daily care during admission, medication instructions and side effects, diet and exercise advice and retest recommendations when discharged.
Intervention fidelity
The principal investigator will be the only interventionist. The principal investigator is a Postdoctoral Fellow in nursing and has received 98 hours of professional training and supervision from experienced ACT therapists. During the intervention process, self-evaluation will be conducted by the interventionist via an ACT core component self-rating form48 (60 items for four sessions, rating scale of 1–7) to assess if each point occurred in the therapy session recording.
The interventionist will participate in semimonthly supervisory meetings with supervisors to develop her skills and ensure treatment adherence to the trial protocol. Obstacles to the participants’ engagement and progress of interventionists and strategies to address these possibilities will be discussed during the meeting. The intervention sessions will be all audiotaped and randomly selected samples of the audiotapes of the intervention will be used by supervisors to give feedback to the interventionist to adjust the skills. A day-to-day self-reflection diary will be used to help the interventionist detect and correct errors during the intervention.
The extent of the ACT implementation will be assessed by retrieving the attendance records of the ACT intervention sessions and the follow-up evaluation.
Outcomes and measures
The primary outcomes comprise of the study feasibility (i.e. eligibility rate, recruitment rate, attrition rate and adherence rate), fatigue interference49–51 of patients and caregiving burden45 52 53 of patients’ primary family caregiver, respectively. The secondary outcomes include HRQOL54 of patients, meaning in life55 56 of caregivers, psychological flexibility57 58 and mindfulness59 60 of both patients and their caregivers. The acceptability of the intervention and participants’ demographic characteristics will also be evaluated. A detailed summary of all outcomes, including the measurements, is presented in table 2.
Descriptions of secondary and functional outcomes measurement tools
Data collection procedure
Data will be collected online at baseline before randomisation (T0) and 1 week postintervention (T1) by completing the online questionnaire link by an independent assessor. The outcomes assessor who is independent of the research will be trained before formally conducting data collecting. A regular audit review in which the researcher and outcome assessor are involved will be conducted every 2 weeks to enhance reliability. The participants in the intervention group will be asked about the intervention acceptability via WeChat on the same day. A sample of 10 dyads in the intervention group will be randomly selected for the focus group interview by the research assistant. The process of data collection is shown in table 3.
Study period for the procedure of enrolment, intervention delivery and data collection
Patient and public involvement
Patients and their primary caregivers were involved in the development of the intervention. Before the start of the study, patients and their primary caregivers were first invited to participate in the main sessions of the study to ensure clarity and comprehension. After the intervention delivery, semistructured interviews will be carried out in participants in the intervention group to explore their experiences on the intervention’s usefulness and acceptability.
Data management
Data collected in the study will be kept confidential and stored in locked filing cabinets and password-protected computers, which are open only to members of the research team. In this study, the intervention booklet and logbook will be delivered to participants to promote their compliance and avoid attrition. On the other hand, the Generalised Estimating Equation (GEE) model will be used to help accommodate missing data and yield relatively few statistical errors when the data are missing at random.61 Data will be destroyed 5 years after completing the study.
Data analysis
IBM SPSS will be realised for data analysis by a statistician blinded to the allocation. Descriptive statistics will be used to calculate continuous data by mean and SD and categorical variables by frequency and percentage. Baseline differences between groups regarding sociodemographics and disease-related data will be examined using a t-test for continuous variables and χ2 test for categorical variables.
All data analysis will be performed according to the Intention-to-Treat principle,62 preserving the integrity of randomisation and minimise selection bias.63 A GEE model will be used to test the preliminary effects of intervention between groups postintervention.61 Effect sizes (Cohen’s d) and a 95% CI will also be reported, and two-tailed p value <0.05 indicates statistical significance. NVIVO V.12 with content analysis64 using an inductive method will be used to explore the ACT experience in patients with advanced lung cancer and caregivers and indicate the intervention acceptability.
Ethics and dissemination
The ethical approval has been obtained from the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (CREC Ref. No. 2023.030) and the Medical Ethics Committee of Xiangya Hospital Central South University (No. 202305336). Written informed consent will be obtained prior to enrolling in the study. Participants will be informed of confidentiality and their right to leave the study at any time and without any penalty. The study conformed to the principles outlined in the Declaration of Helsinki. The findings will be disseminated in peer-reviewed journals and through local or international conference presentations.
Discussion
This pilot study focuses on the major concern (i.e. fatigue interference) of patients with advanced lung cancer and caregiving burden of their family caregivers. ACT, as a novel intervention, shows promises in addressing these primary concerns for advanced lung cancer patient–caregiver dyads. The findings of the study will help provide initial evidence of the online-based ACT intervention’s feasibility, acceptability and potential for generating meaningful effects.
This is one of the few studies29 65 to test the feasibility of preliminary effects of ACT in advanced cancer patient–caregiver dyads. Our previous RCT has shown promising evidence of ACT on fatigue interference in patients with advanced lung cancer.41 The current study builds on prior work by examining both advanced lung cancer patients with fatigue interference and the caregiving burden of their caregivers. By receiving ACT intervention, patients with advanced lung cancer and caregivers will be more flexible to cope with the fatigue interference and caregiving burden, increase tolerance to unwanted thoughts and emotions, thus reducing the use of medical resources in the long-term period and thus reduce direct medical expenditure. This dyadic ACT intervention may also benefit both patients and caregivers by capitalising on their existing familial relationship.12 This can facilitate shared learning and mutual support during the intervention and ongoing self-practice processes, ultimately enhancing their overall quality of life.12
It is worth noting that this study focuses on the most urgent problems for patients with advanced lung cancer need to solve: CRF and develops an online-based intervention suitable for people with Chinese background for this specific symptom living in rural areas. The online-based ACT intervention can often be more affordable compared with traditional in-person therapy sessions, making it a more accessible option for patients with limited financial resources. The online mode also can ensure continuity of care, allowing patients to maintain regular psychological support without interruption, regardless of their location. This can be particularly beneficial for patients living in remote areas, where accessing in-person follow-up care may be challenging.31 In addition, VR technology supported mindfulness exercise is adopted as one of the strategies to facilitate the intervention process. Participants can practice VR supported mindfulness exercises anywhere, as long as they have a compatible smartphone and VR headset. Mobile VR harnesses the smartphones with mindful animations and combines them with VR headsets to provide participants with immersive and interactive VR experiences. With high-quality visuals, head tracking and various interaction methods, participants will be able to focus on the present moment and intervention process.
Several limitations should be noted. First, the participants will be recruited from a hospital in central China, which will limit the generalisability of the study findings. Second, due to the nature of the intervention, blinding of participants and the interventionist will be impractical. Third, the small sample size may impact statistical power; however, the primary objective of this study is to assess the feasibility and initial effects of the online-based intervention before conducting a fully powered trial.
Overall, the results will provide a foundation for a full-scale RCT focused on the novel application of online-based ACT to fatigue interference and caregiver burden in advanced cancer in rural China. On the other hand, this study will further respond to national policies and strive to give everyone the equal right to participate in psychological interventions and maintain mental health, thus ultimately realising the vision of national mental health in China.
Supplemental material
Ethics statements
Patient consent for publication
Acknowledgments
This work was supported by the Association for Contextual Behavioural Science (ACBS) Research Development Grant (Grant number: N/A) from the Association for Contextual Behavioural Science. The funder was not involved in the study design and will not contribute to data collection, analysis, interpretation of data or manuscript writing and the decision to submit the report for publication and will not have ultimate authority over any of these activities. The authors are grateful to the senior statistician in the department for statistical guidance.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors HL and CLW contributed to the conception and design of the trial and acted as guarantors. XJ provided expert advice and commented on the intervention contents. CLW and XJ made recommendations on qualitative design and analysis. NW and ZS provided advice on the protocol and coordinated participant recruitment and the intervention procedure. HL and CLW wrote the manuscript, and all authors revised and approved the manuscript.
Funding This work was supported by the Association for Contextual Behavioural Science (ACBS) Research Development Grant (Grant number: N/A), ACBS, USA.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.