Article Text
Abstract
Introduction Benign prostatic hyperplasia (BPH) is a condition commonly seen among men aged over 40, significantly affecting their quality of life and typically accompanied by lower urinary tract symptoms (LUTS). Acupuncture presents a potentially effective treatment option; however, the exact effects remain uncertain. Therefore, we design this multicentre randomised trial to evaluate the efficacy and safety of electroacupuncture (EA) for relieving LUTS in men with BPH.
Methods and analysis A two-arm, sham-controlled, subject-blinded and assessor-blinded trial will be conducted in 11 hospitals in China to compare EA with sham electroacupuncture (SA) in treating moderate to severe LUTS of BPH among men aged 40–80. A total of 306 eligible male patients will be recruited and assigned at a 1:1 ratio to receive either EA or SA for 24 sessions over a succession of 8 weeks, with 24 weeks of follow-up. The primary outcome will be the proportions of participants with at least 30% reduction in the International Prostate Symptom Score total score from baseline at weeks 8 and 20. All statistical analyses will be conducted in accordance with the intention-to-treat principle, and a two-tailed p value less than 0.05 will be considered statistically significant.
Ethics and dissemination The trial has been approved by the institutional review board of Guang’anmen Hospital (2022-203-KY), as well as other recruitment centres. Each participant will receive the detailed information of the trial, and sign the written informed consent. The results of the trial are expected to be published in a peer-reviewed journal.
Trial registration number NCT05585450.
- Clinical Trial
- Prostate disease
- Prostate
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is the first strictly designed multicentre, randomised, sham-controlled trial to evaluate the efficacy and safety of electroacupuncture for lower urinary tract symptoms in men with benign prostatic hyperplasia.
To ensure successful blinding in this trial, needling in sham acupoints with superficial penetration and minimal electric current for 30 s is designed for sham acupuncture group.
Bias may occur as acupuncturists will be aware of the treatment allocation.
Introduction
Benign prostatic hyperplasia (BPH) is a common disorder that affecting about 36.6% of men aged over 40 years in China.1 2 Shown in histological findings, BPH is characterised by an increase of both stromal and epithelial cells in the transitional zone of prostate, which surrounds the urethra. This leads to urethra compression and resistance to urine flow, as well as obstruction-induced functional changes in bladder, termed benign prostatic obstruction (BPO), such as overactivity and reduced contractility of the detrusor muscle.3 According to the European Association of Urology (EAU) guideline, there is a growing tendency to avoid using the term BPH to describe lower urinary tract symptoms (LUTS) that are actually a consequence of BPO.4 LUTS encompass a spectrum of symptoms including urine urgency, frequency, nocturia, dysuria, hesitancy, intermittency and incomplete bladder emptying, which severely affect patients’ quality of life (QoL), disrupting sleep patterns or interfering with daily activities.5
Options of the treatment to LUTS in men with BPH range from watchful waiting to medical and surgical interventions, depending on the severity of the symptoms and the level of discomfort.4 Effective medical therapy typically involves both α-adrenergic blockers and 5α-reductase inhibitors (5-ARIs); however, these medications may cause side effects, such as asthenia, dizziness, orthostatic hypotension (α-adrenergic blockers)6 and reduced libido and erectile dysfunction (ED) (5-ARIs).7 Unfortunately, it remains uncertain whether alternative medications, including plant extracts, are effective.4 In cases where conservative therapy fails or urinary retention relapses, surgical interventions, such as transurethral resection of the prostate, may be recommended.8 9 However, such procedures of surgery present potential risks, including retrograde ejaculation, ED, haematuria and urinary tract infection,10 where approximately 5%–10% of the postsurgery patients require repeated surgery within 10 years.11 In view of the drawbacks of the medical and surgical interventions, alternative therapies of efficacy and safety are urgently needed.
A series of studies have suggested that acupuncture is an effective treatment option for urological conditions, including urinary incontinence12 13 and chronic prostatitis/chronic pelvic pain syndrome.14 According to our previous studies,15 as well as the recent researches,16 17 and a systematic review,18 acupuncture may relieve LUTS and improve QoL in patients with BPH. However, the effects of acupuncture remain uncertain due to small sample sizes, and lack of proper designs. Therefore, we intend to design and conduct this randomised controlled trial to evaluate the efficacy and safety of electroacupuncture (EA) in relieving LUTS in men with BPH.
Methods
Study design
This multicentre, randomised, sham-controlled, subject-blinded and assessor-blinded trial will be performed at 11 hospitals in China, which are Guang’anmen Hospital, Acupuncture and Moxibustion Hospital of China Academy of Chinese Medical Sciences (CACMS), Affiliated Hospital of Nanjing University of Chinese Medicine, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, The First Affiliated Hospital of Anhui University of Chinese Medicine, The First Affiliated Hospital of Hunan University of Chinese Medicine, West China Hospital of Sichuan University, The Second Affiliated Hospital of Guiyang University of Traditional Chinese Medicine, Jinan Hospital of Traditional Chinese Medicine, Qingdao Hospital of Traditional Chinese Medicine and Yantai Hospital of Traditional Chinese Medicine.
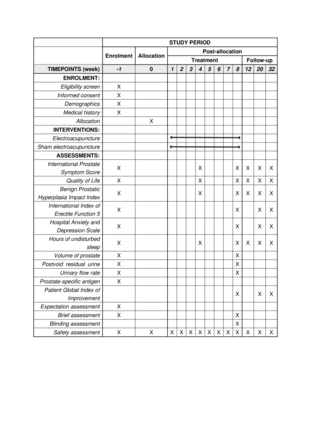
This design and protocol were developed in accordance with the guidelines for clinical trials and the standards for reporting interventions in acupuncture-based clinical trials. The study was approved by the institutional review boards at the coordinating centre (ethical approval number: 2022-203-KY) and other study centres, and it has been registered at ClinicalTrials.gov on 18 October 2022. Two parallel-arm groups, the EA group and the sham electroacupuncture (SA) group, are comprised within the framework of this trial. The study will span over a period of 33 weeks for each participant, which includes a baseline week before randomisation, 8 weeks of treatment and 24 weeks of follow-up (figure 1).
Study flowchart. BPH, benign prostatic hyperplasia.
Recruitment
The planned start date was 20 October 2022; however, due to the COVID-19 pandemic, actual enrolment began on 9 March 2023. The estimated end date is 30 December 2025. A total of 306 male participants will be recruited through various public advertisements, such as posters, hospital websites and WeChat public accounts. Urologists will be in charge of the screening and diagnosis, and conduct an array of evaluation, including a detailed medical history, physical examinations (mainly digital–rectal examination), urinalysis, ultrasound for the prostate, postvoid residual (PVR) urine, uroflowmetry and prostate-specific antigen (PSA). The research assistants will inform all participants with a thorough explanation of the potential benefits and risks associated with this trial, the randomised allocation of treatments and the enrolment. Each participant will sign a written informed consent before the enrolment and has the right to withdraw from the trial at any time.
Inclusion criteria
Participants will be included if they have:
Diagnosis for LUTS attributed to BPH in accordance with the guidelines of EAU4 and American Urological Association (AUA).19
Men aged between 40 and 80 years.
LUTS due to BPH for at least 3 months.
International Prostate Symptom Score (IPSS) total score≥8.
Prostate volume≥20 mL.
Maximum urinary flow rate (Qmax)≤15 mL/s.
Voluntarily participate in the trial and sign the written informed content.
Exclusion criteria
Participants will be excluded if they have:
PVR volume≥150 mL.
Acute urinary retention or catheterisation within the 3 months.
Prostate cancer or PSA level≥4.0 ng/mL.
Neurogenic lower urinary tract dysfunction; prostatitis; urinary tract infections; urethral strictures; bladder diverticula; bladder stones; bladder cancer; history of genitourinary system surgery (prostate, bladder, urethra, etc).
Previous acupuncture treatment for BPH in the preceding 1 month, or usage of α-blockers, 5-ARIs, muscarinic receptor antagonists or any other specific medication in the previous 2 weeks unless a stable 5-ARIs usage of over 3 months.
Severe lung, heart, liver, kidney, metabolic or mental illness, coagulation dysfunction or with obvious cognitive dysfunction.
Installed cardiac pacemaker, allergy to metal, severe fear of acupuncture or unbearable to the stimulation of EA.
Randomisation and blinding
The allocation sequence will be generated independently by Lnkmed Tech Co. (Beijing, China). Eligible participants will be randomly assigned in a 1:1 ratio to either the EA group or the SA group using both stratification by site and permuted blocks with random block sizes. Research assistants who are not engaged in intervention and evaluation will have access to the participant allocation information via a central randomisation system. The treatment allocations will be concealed from the participants, outcome assessors and statisticians to ensure blinding.
Intervention
EA group
The acupoint protocol is based on the meridian theory of traditional Chinese medicine, the results of previous studies,15 and the consensus of experienced acupuncturists from CACMS. Participants in the EA group will receive treatment at bilateral Bladder Meridian 32 (BL32, Ciliao), BL33 (Zhongliao), BL35 (Huiyang) and Spleen Meridian 6 (SP6, Sanyinjiao). BL32 and BL33 are located in the second and third posterior sacral foramen, respectively; BL35 is located 0.5 cun (≈10 mm) lateral to the extremity of the coccyx; SP6 is located posterior to the medial border of the tibia and 3 cun (≈60 mm) superior to the prominence of the medial malleolus.
BL32 and BL33 will be inserted by needles of 0.30×75 mm size at an angle of 50°−75°, inward and downward, to a depth of 60–70 mm. BL35 will be inserted by the same size needles, slightly outward and upward, to a depth of 60–70 mm. SP6 will be inserted vertically by needles of 0.30×40 mm to a depth of 25–30 mm. After insertion, the needles located at BL35 and SP6 will be lifted, thrust and twisted evenly three times to induce the sensation of deqi. The EA therapeutic apparatus (Yingdi KWD 808I electro pulse acupuncture therapeutic apparatus, Changzhou Yingdi Electronic Medical Device Co.) will be connected transversally to four pairs of needles, with a continuous wave of 5 Hz and an electric current ranging from 0.5 to 4 mA for 30 min, depending on the participant’s comfort level.
SA group
Participants in the SA group will receive superficial needling at bilateral non-acupoints lateral to the corresponding acupoints (2 cun (≈40 mm) horizontally outside BL32, BL33, and BL35; sham SP6, in the middle of SP6 and tendons). The four pairs of non-acupoints will be inserted by needles of 0.30×25 mm or 0.30×40 mm size to a depth of 2–3 mm until the needles can stand still. No manipulation will be performed, and the sensation of deqi will not be induced. The same EA therapeutic apparatus will be connected transversally to four pairs of needles, with a continuous wave of 5 Hz and a minimal electric current ranging from (ideally at a degree which participant can just perceive). After 30 s, the electric current will be turned down, leaving the indicator light, and ticking sound on.
The treatment in both groups will last 30 min for each session, three sessions per week (ideally every other day) for a succession of 8 weeks. At least 2 acupuncturists who had 5-year undergraduate education in acupuncture and more than 2-year clinical experience will administer treatment at each centre. To guarantee the consistency in treatments, acupuncturists will receive standardised operation procedure training before conducting treatments. This training includes a video tutorial that will provide detailed information on how to perform both EA and SA correctly.
The administration of medications or other therapies for LUTS will be discouraged throughout this trial unless the symptoms become intolerable. However, the stable usage of a 5-ARIs for over 3 months is deemed permissible. The treatment details will be recorded accordingly, including the name and the duration.
Outcomes
Primary outcome
The two coprimary outcomes include the proportions of participants with at least 30% reduction in the IPSS total score from baseline at weeks 8 and 20.
Secondary outcomes
Secondary outcomes will be measured by a range of tools, including the IPSS total score and subscales of voiding, storage and numbers of nocturia, the IPSS QoL, the BPH Impact Index (BPH-II) and hours of undisturbed sleep (HUS) at weeks 4, 8, 12, 20 and 32; the International Index of Erectile Function 5 (IIEF-5), the Hospital Anxiety and Depression Scale (HADS) and the Patient Global Index of Improvement (PGI-I) at weeks 8, 20 and 32. The volume of the prostate and PVR urine, maximum and average flow rate will also be measured at week 8 (figure 2). The secondary outcome measures and the time frame are shown in table 1.
Study Schedule.
Secondary outcome measures
The IPSS is a 7-item, reliable, valid and sensitive questionnaire that is commonly used to assess the severity of LUTS, including filling (urgency, frequency and nocturia) and voiding (incomplete emptying, intermittency, straining and weak urinary stream) symptoms.20–22 The score of IPSS ranges from 0 to 35, with scores of 0–7 indicating mild symptoms; 8–19 indicating moderate symptoms and 20–35 indicating severe symptoms.22 It has been established that a decrease of at least three points is the minimal clinically important difference,23 while a 30% reduction in the IPSS total score is the minimal clinical improvement recommended by the US Food and Drug Administration (FDA) for device therapy.24
The IPSS QoL includes only one specific question: if you are to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that? The response is categorised into seven levels, with a score ranging from 0 to 6, and higher scores indicating poorer QoL. Despite its simplicity, this question is strongly associated with the overall symptom score.25
The BPH-II is a 4-item, self-administered tool that measures the interference of LUTS in participants’ physical, mental and usual activities over the past month. The score of BPH-II ranges from 0 to 13, with higher scores indicating greater BPH symptom-related impact.26
The IIEF-5 is an abridged, 5-item instrument for evaluating erectile function, ranging from 1 to 25 (normal, 22–25; mild, 17–21; mild to moderate, 12–16; moderate, 8–11 or severe, 1–7).27 28
The HUS is defined as the duration from falling asleep till awake in the morning, or till the first nocturia if any.29
The HADS is developed to quantify psychological distress, consisting of two 7-item subscales, one for anxiety and one for depression. The total score ranges from 0 to 42, with higher scores indicating worse conditions.30
The PGI-I evaluates the overall treatment effect as perceived by the participants themselves. The change can be rated in seven levels, including ‘very much better’, ‘much better’, ‘a little better’, ‘no change’, ‘a little worse’, ‘much worse’ or ‘very much worse’.31
Expectation and brief assessment
To assess participants’ expectations of improvement in LUTS, participants will be asked: how do you expect the LUTS to be in 8 weeks at baseline. To assess participants’ belief of EA, at both baseline and week 8, participants will be asked: do you think that EA may be beneficial in treating your BPH?
Blinding assessment
Participants will be informed that there is a 50% chance of being allocated to receive either the traditional EA with deeper needling or the SA with shallower needling. After the last session at week 8, each participant will be asked whether they have received traditional EA, with the option of ‘Yes’ or ‘No’.
Safety assessment
EA-associated adverse events, such as bruising, haematomas, infection or numbness as well as any other adverse events unrelated to EA, will be carefully documented. Serious adverse events will be reported to the institutional review boards of Guang’anmen Hospital within 24 hours.
Data management and quality control
To ensure the consistency, personnel in each recruitment centre will receive extensive training from the principal investigator (ZL) on details of the protocol.
All treatments for each participant will be completed by 1–2 specific acupuncturists. In addition, one assessor must maintain responsibility for the same participants throughout the trial. They will explain the contents of handbook, if necessary, as well as remind the participants of their schedule through either phone or WeChat. At each assessment visit, the data will be collected and recorded in the paper case report form (CRF) promptly by assessors. The clinical research coordinators will type the data into the electronic data capture (EDC) system within 1 week. The clinical research associates (CRA) will supervise weekly through the system to enhance the quality. All data on the EDC system will be locked on verification of consistency between data online and the paper CRFs by two independent CRAs.
All deviations from the study protocol will be reported in time. Participants who withdraw or drop out will be documented during the trial. Lnkmed Tech Co. (Beijing, China) will be responsible to conceal the treatment allocation, which will only be revealed after the statistical analysis is completed.
Statistical methods
Sample size
To estimate the sample size, we will assume the proportions of participants with at least 30% reduction in the IPSS total score from baseline at week 8 to be 75% in the EA group and 55% in the SA group based on the results of our unpublished study, which showed that the primary outcome at week 8 was 77% among the group receiving EA and 55% among the SA group. The study needed 236 participants to achieve 90% power with a two-sided α level of 0.05. Assuming a 20% dropout or withdrawal rate, the study will need 306 participants to provide 90% power with a two-sided α level of 0.05.
Statistical analysis
The two null hypotheses are that EA will be the equal to SA at both weeks 8 and 20, and as well as week 32. The primary outcome will be analysed using a generalised linear model with a binomial distribution and identity link. Changes from baseline in the IPSS total score will be analysed using a mixed-effects models for repeated measures. The observed change from baseline at each visit will be considered as the dependent variable. The same approach will be used in other longitudinal continuous outcomes, such as IPSS subscales (filling and voiding), number of nocturia and BPH-II scores. The PGI-I, participants’ expectations and brief assessment, adherence, blinding and adverse event data will be provided for descriptive purposes only.
Multiplicity on the primary outcome will be controlled by a closed testing procedure.32 In the closed testing procedure for the primary outcome, EA and SA will only be compared at week 32 when the comparisons between EA and SA have to be positive (p-value lower than 0.05) at weeks 8 and 20. Secondary analyses will be considered supportive in nature and will be not controlled for multiplicity. The sensitivity analysis of the primary outcome will be repeated using two analytical approaches. First, multiple imputation will be used to impute missing IPSS total score. Second, the baseline usage of the 5-ARIs will be used as a covariate in the primary analysis.
All analyses will be conducted using SAS V.9.4 (SAS Institute) in accordance with the intention-to-treat principle, and a two-tailed p value less than 0.05 will be considered statistically significant.
Patient and public involvement
During the conception period of the study, we conducted interviews with a subset of BPH patients with LUTS. This allowed us to gather insights into the impact of primary symptoms on QoL, changes observed after EA, as well as the acceptance and perspectives on EA. These insights played a crucial role in later study design, particularly in determining the target population and selecting outcome measures. Patients and/or the public were not involved in the recruitment and conduct of this study. Patients who actively contributed to the consultation process for the trial design will be excluded. The results of the study will be communicated in plain language and disseminated to the public, including participants, through various public and social media channels. Participants will receive the study intervention free of charge during the study period.
Ethics and dissemination
The trial has been approved by the institutional review board of Guang’anmen Hospital (2022-203-KY), as well as other recruitment centres, and will be conducted in accordance with the Declaration of Helsinki. Each participant will receive the detailed information of the trial, and sign the written informed consent (online supplemental material). Those in the SA group will be compensated with 24-session EA treatment. The results of the trial are expected to be published in a peer-reviewed journal.
Supplemental material
Discussion
Patients with BPH can be managed with watchful waiting when no complications set in and IPSS≤7, although histological evidence and enlarged prostates may exist.4 However, patients with moderate to severe BPH may suffer in daily activities and face huge financial burden.5 33 In addition, low Qmax may indicate detrusor underactivity,34 and Qmax less than 15 mL/s may indicate bladder outlet obstruction, which sensitivity was tested 82%35 and poorly relieved by ablative technique, a minimal invasive treatment.4 Whereas EA could alleviate LUTS by augmenting detrusor contractions and diminishing obstructions.36 Therefore, this study will focus on patients with LUTS lasting more than 3 months, IPSS score over eight points, and Qmax≤15 mL/s.
Medical therapy, such as 5-ARIs, could reduce prostate volume and slow down the progression of the disease, with gradual effects, taking as long as 3–6 months to respond. As the long-term of usage of the medication might lead to unacceptable side effects, like ED,37 many patients in China turned to acupuncture treatment, a complementary and alternative therapy that is effective and safe in public view. This study will adopt standardised acupuncture scheme based on the meridian theory and clinical experiences. Stimulation at the acupoint of SP 6, which is located over the posterior tibial nerve and is the crossroad of intersection of the Spleen, Kidney and Liver Meridians, has been found beneficial in relieving LUTS.38 39 Similarly, the acupoints along Bladder Meridian, such as BL32 and BL33, have been regularly used to address urologic disorders, for the acupoints are located in the sacral hiatus where nerves of loin and sacrum traverse and the stimulation could benefit LUTS.40 41 However, it is challenging to set up an ideal sham control in acupuncture clinical trials. To ensure successful blinding in this trial, needling in sham acupoints with superficial penetration and minimal electric current for 30 s is designed for SA group where therapeutic effects may present nevertheless.42
The hypothesis of this trial is that EA is superior to SA in relieving LUTS in patients with moderate-to-severe BPH. The efficacy will be mainly reflected in the proportions of patients whose IPSS total score is reduced by 30% or more from baseline, a level of the minimal clinical improvement recommended by the US FDA for device therapy.24 Based on our clinical experience and unpublished pilot study, this trial will select weeks 8 and 20 as the primary outcome timepoints to evaluate the immediate effects after 8 week treatment and the sustained effects after 12 week cessation of treatment. Furthermore, to provide deeper insights into its clinical significance, an extended long-term follow-up will be conducted at week 32, which serves as the key secondary outcome timepoint.
Although the study will intend to provide robust evidence on efficacy and safety of EA in treating BPH by blinding outcome assessors and patients, bias could occur as acupuncturists will be aware of the treatment allocation. In addition, the results of this trial may not be generalised globally as the trial will be performed in China only.
Ethics statements
Patient consent for publication
Acknowledgments
We are deeply grateful to all personnel in recruitment centres for their invaluable contributions. We also extend sincere appreciation to all the patients, particularly the patient advisers who have generously shared their insights to enhance the study design.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
LZ and YY contributed equally.
Contributors LZ, YY and ZL: conceived and designed the experiments; wrote the paper. JY, YS, YC and JF: performed the experiments; wrote the paper. YL: analysed and interpreted the data; wrote the paper. All authors have read and approved to the final version. ZL is responsible for the overall content (as guarantor).
Funding This work was supported by Scientific and technological innovation project of China Academy of Chinese Medical Sciences (grant number: CI2021B012), High Level Chinese Medical Hospital Promotion Project (grant number: HLCMHPP2023089) and Guang’anmen Hospital, China Academy of Chinese Medical Sciences (grant number: 2022079).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.