Article Text
Abstract
Objectives The economic implications of combining rezvilutamide with androgen deprivation therapy (ADT) remain uncertain, despite the observed survival advantages compared with bicalutamide plus ADT. Therefore, this study evaluates the cost-effectiveness of rezvilutamide plus ADT as the first-line treatment of metastatic hormone-sensitive prostate cancer (mHSPC) from the perspective of the Chinese healthcare system.
Design A partitioned survival model was developed to assess the cost-effectiveness of rezvilutamide combined with ADT. Clinical data were obtained from the CHART trial. Costs and utility values were obtained from local estimate and published literature. Only direct medical costs were included in the model.
Interventions Rezvilutamide was administered at 240 mg daily or bicalutamide at 50 mg daily until progression.
Outcome measures The main outputs of the model included costs and quality-adjusted life years (QALYs), which were used to determine the incremental cost-effectiveness ratio (ICER). One-way and probabilistic sensitivity analysis (PSA) were used to explore model uncertainties.
Results The rezvilutamide group showed an expected gain of 2.28 QALYs and an incremental cost of US$60 758.82 compared with the bicalutamide group. The ICER for rezvilutamide group versus bicalutamide group was US$26 656.94 per QALY. The variables with the greatest impact on the model results were the utility for progression-free survival state and the price of rezvilutamide. PSA revealed that rezvilutamide group had 100% probability of being cost-effective at a willingness-to-pay threshold of US$35707.5 per QALY.
Conclusion Rezvilutamide in combination with ADT is more cost-effective compared with bicalutamide plus ADT as the first-line treatment of mHSPC from the perspective of the Chinese healthcare system.
- prostatic neoplasms
- health economics
- public health
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Partitioned survival model, a most widely accepted modelling approach in oncology, was used to evaluate the cost-effectiveness, with inclusion of uncertainty and robustness assessment.
The study was based on the CHART trial, which was a high-quality phase III randomised controlled trial.
External validation of the overall survival modelling has been performed using parameter distribution fitting.
Some parameters such as utility and cost of adverse events-related treatments were derived from the published literature that may not be represented.
Introduction
Prostate cancer is the second most commonly diagnosed cancer and ranks fifth among cancer-related deaths in men worldwide, accounting for 14.1% of all new cancer diagnoses and 6.8% of all cancer-related deaths in men worldwide.1 Although the incidence of prostate cancer is relatively low being the sixth frequently diagnosed cancer in Chinese males, China accounts for 8.2% of the global new cases of prostate cancer due to its large population.2 The low incidence rate may be attributed to the lack of widespread prostate cancer screening in China. Only 40% of newly diagnosed men have localised prostate cancer in China,3 compared with approximately 83% in the USA.4 The 5-year survival rate of patients with metastatic disease is approximately 30%.5 Therefore, innovative drugs have received a lot of attention aiming to improving survival of metastatic prostate cancer.
Metastatic hormone-sensitive prostate cancer (mHSPC) is defined as a metastatic disease in patients who have either not received or continue to respond to primary hormone therapy.6 While androgen deprivation therapy (ADT) remains the mainstay of treatment in patients with mHSPC, the addition of antiandrogen drugs has become the preferred therapy option, including first-generation antiandrogens (eg, bicalutamide), second-generation antiandrogens (eg, enzalutamide, apalutamide and darolutamide) and the androgen biosynthesis inhibitor abiraterone.7–9
Rezvilutamide, a novel second-generation antiandrogen, was approved in China in June 2022 for the treatment of patients with mHSPC. It has higher androgen receptor (AR) inhibitory activity without AR partial agonism.10 The CHART trial showed that rezvilutamide in combination with ADT significantly improved the overall survival (OS) and reduced the risk of death in patients with high-volume mHSPC compared with bicalutamide plus ADT (HR 0.58 (95% CI: 0.44 to 0.77)).11
The rezvilutamide plus ADT is an attractive therapeutic option that significantly decreases the risk of cancer progression and death among patients with mHSPC. However, the high price of rezvilutamide (US$822.5 per month) lead to a heavy economic burden for patients. It is unclear whether rezvilutamide plus ADT is cost-effective as a first-line treatment for patients with mHSPC for now. Thus, the objective of this study is to investigate the pharmacoeconomic profile of rezvilutamide plus ADT in comparison with bicalutamide plus ADT for patients with high-volume mHSPC from the perspective of the Chinese healthcare system.
Methods
Patients and interventions
The model was constructed using the sample from the CHART trial, which is a prospective, randomised, open-label, phase III clinical trial.11 Eligible patients included men aged 18 years or older with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and had high-volume metastatic, hormone-sensitive prostate cancer. Patients were randomly assigned (1:1) to receive ADT plus either rezvilutamide (240 mg) or bicalutamide (50 mg) orally once daily via an interactive response technology system with a block size of four. Randomisation was stratified according to ECOG performance status and presence of visceral metastasis. The trial was conducted between 28 June 2018 and 6 August 2020.
Model structure
A partitioned survival model (PSM) was constructed using Microsoft Excel to compare the long-term health and cost outcomes of patients with mHSPC from the perspective of the Chinese healthcare system. PSM is the most frequently used approach in the economic evaluation of cancer treatments according to a review of the National Institute for Health and Care Excellence appraisals.12 The model included three health states: progression-free survival (PFS), progressed disease (PD) and death (online supplemental figure 1). On the commencement of treatment, all patients were assumed to have entered a PFS state. Patients transitioned to PD and death state according to the trial data.
Supplemental material
According to the National Bureau of Statistics of China, the average life expectancy for residents in 2020 was 77.9 years,13 considering that the median age of patients in the CHART study was 69 years, with an age range of 64–74 years.11 Therefore, we have decided to adopt a 20-year model time to more accurately reflect the long-term survival of patients. The cycle length was set to 28 days, which was consistent with the length of the treatment periods. The health utilities for PFS and PD were derived from previous studies (table 1). Total costs, quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICERs) are the output data obtained from our model. ICER was calculated by dividing the change in costs between the intervention and control group by the change in QALY. All costs and health outcomes were discounted at 5%.14 It is suggested that the willingness-to-pay (WTP) threshold for QALYs should be set at 1–3 times the national per capita gross domestic product (GDP) according to the Chinese Pharmacoeconomic Evaluation Guidelines.14 If ICER>3 times GDP per capita, the intervention strategy is considered not cost-effective. In this study, we set the WTP threshold at US$35 707.5/QALY (three times the per capita GDP in China in 2022).
Key model input parameters
Clinical data
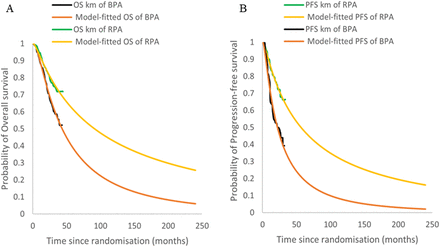
Survival data were collected from the survival curves derived from the CHART study using Web-Plot Digitizer. Standard parametric models15 (eg, Weibull, exponential, log-normal, log-logistic and Gompertz) were examined in R V.3.6.3 to select the best-fit survival functions using the Akaike information criterion (AIC), Bayesian information criterion (BIC) and visual inspection for parametric extrapolation and long-term survival estimates. Lower AIC and BIC values indicated a better fit of the selected model. The log-normal model was selected as the best-fit model for the OS and PFS curves of the rezvilutamide plus ADT and bicalutamide plus ADT groups (online supplemental table 1). The parameters of the log-normal survival function are listed in online supplemental table 2. Superimposed graphs of the Kaplan-Meier curves from the trial and the predicted curves based on the best-fitting parametric survival models are presented in figure 1.
Model outcomes compared with digitised Kaplan-Meier (KM) data for (A) OS KM curves and fitting curves of RPA and BPA and (B) PFS KM curves and fitting curves of RPA and BPA. BPA, bicalutamide plus androgen deprivation therapy; OS, overall survival; PFS, progression-free survival; RPA, rezvilutamide plus androgen deprivation therapy.
Costs
The direct medical costs were covered, including drug costs, costs of adverse events (AEs) management, costs of subsequent treatment and costs of disease management. The proportion of patients who underwent surgical ADT (orchiectomy) in the rezvilutamide and bicalutamide groups was 3% and 5%, respectively.11 The remaining patients were treated with luteinising hormone-releasing hormone (LHRH) therapy. The cost of LHRH therapy was estimated using Leuprorelin Acetate Microspheres for Injection (Enantone) in a 3-month dose of 11.25 mg.16 Costs of subsequent treatment were estimated based on clinical expert consensus, and the treatment regimen includes Abiraterone Acetate Plus Prednisone (COU-AA-302 Study)17 and enzalutamide (PREVAIL Study).18
In addition, we acquired the drug and administration costs from local estimate (Zhejiang Provincial Centre for drug & Medical Device Procurement) and retrieved costs of AE-related treatments and orchidectomy from previously published studies (table 1).19 All costs were converted into US dollars (US$/¥=7.2).
Sensitivity analysis
One-way sensitivity analysis and probabilistic sensitivity analysis (PSA) were conducted to examine model uncertainty. One-way sensitivity analysis was performed to evaluate the effects of each parameter on the model. The estimated range of each variable was determined by assuming a 20% change from the baseline value (table 1) and the discount rate ranged from 0% to 8%. Results are displayed in tornado diagrams based on the impact of the parameters on the ICER. In the PSA, we performed Monte Carlo simulations with 1000 iterations through random sampling from the assigned distributions (table 1). The PSA results were represented using a scatter plot of incremental cost-effectiveness and cost-effectiveness acceptability curves.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Base-case analyses
Over the 20-year period, rezvilutamide plus ADT accrued costs of US$106 807.74 with 6.08 QALYs, while bicalutamide plus ADT yielded cost of US$46 048.92 with 3.80 QALYs (table 2). These data translated to an ICER of US$26 656.94/QALY, indicating that rezvilutamide combined with ADT is cost-effective compared with bicalutamide plus ADT for the first-line treatment of mHSPC from the perspective of the Chinese health system at a WTP threshold of US$35 707.5/QALY.
Summary of base-case analyses
Sensitivity analyses
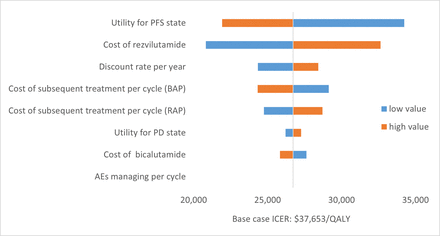
Results of the one-way sensitivity analysis were presented in a tornado diagram (figure 2). The utility of the PFS state showed the strongest impact on the model results, followed by the price of rezvilutamide and the discount rate. As each parameter varied across broad ranges, the ICER results for the one-way sensitivity analysis ranged from US$20 746.54 to US$34 158.44 per QALY gained, which was lower than the WTP value. The PSA results suggested that the probability of rezvilutamide group being cost-effective compared with bicalutamide group was 100% at a WTP threshold of US$35 707.5/QALY (figure 3).
Tornado diagrams of one-way sensitivity analyses. AEs, adverse events; BPA, bicalutamide plus androgen deprivation therapy; ICER, incremental cost-effectiveness ratio; PD, progressed disease; PFS, progression-free survival; QALY, quality-adjusted life years; RPA, rezvilutamide plus androgen deprivation therapy.
Probabilistic sensitivity analysis: (A) incremental cost-effectiveness scatter plot and (B) cost-effectiveness acceptability curves. BPA, bicalutamide plus androgen deprivation therapy; QALY, quality-adjusted life years; RPA, rezvilutamide plus androgen deprivation therapy; WTP, willingness-to-pay.
Discussion
The CHART trial showed that rezvilutamide in combination with ADT significantly improved overall survival and reduced the risk of death by 42% in patients with mHSPC compared with bicalutamide plus ADT.11 Based on data from the CHART trial, this study evaluated the pharmacoeconomic profile of rezvilutamide plus ADT compared with bicalutamide plus ADT in the treatment of patients with mHSPC in the Chinese healthcare system. Our findings suggested that, rezvilutamide plus ADT has become a cost-effective treatment option compared with bicalutamide plus ADT from the perspective of the Chinese healthcare system. Sensitivity analyses verify the robustness of the proposed model.
According to the guideline of the Chinese Society of Clinical Oncology for prostatic cancer, the recommended first-line treatment for patients with mHSPC is ADT plus an antiandrogen such as bicalutamide, rezvilutamide, abiraterone, enzalutamide or apalutamide.20 Sung et al 21 compared the cost-effectiveness of first-line treatment options for mHSPC from the perspective of US payers, including ADT alone or ADT plus one of the following drug: docetaxel, abiraterone, enzalutamide or apalutamide. This study suggests that abiraterone plus ADT is the preferred treatment option for patients with mHSPC, at a WTP threshold of US$100 000 pre QALY. Barbier et al 22 also concluded that ADT combined with abiraterone was more cost-effective than ADT plus apalutamide or enzalutamide for mHSPC from the perspective of Swiss payers. However, ADT plus docetaxel is a more cost-effective strategy than ADT plus abiraterone acetate in patients with mHSPC from the societal perspective of Hong Kong.23 Rezvilutamide is an innovative second-generation antiandrogen; however, its economics have not yet been clarified. This study is the first economic evaluation to compare the cost-effectiveness of rezvilutamide as a first-line treatment for patients with mHSPC from the perspective of the Chinese healthcare system, thus providing new evidence for clinical decision-making regarding antiandrogen drugs for the Chinese patients with mHSPC.
The results of the one-way sensitivity analysis illustrated that the ICER was sensitive to the cost of rezvilutamide. Before price negotiations, the cost of rezvilutamide was US$2 722.22 and the ICER was US$82 564.65 per QALY, which was not cost-effective compared with the bicalutamide group. Fortunately, rezvilutamide is covered by the National Health Insurance from March 2023 after reaching a price agreement with the National Healthcare Security Administration. Owing to a 70% reduction in the retail price, rezvilutamide became a cost-effective option, with an ICER of US$26 656.94 per QALY. It is well known that the launch prices of new anticancer agents are usually high, and national drug pricing negotiations are an effective way to lower these prices and make them more cost-effective. In China, national drug pricing negotiations began in 2016, with drug categories gradually expanding from three at the beginning to 121 in 2023; the price of drugs was reduced by 60.1% on average after drug price negotiations in 2023, thus greatly improving the economics of drug therapy.
This study has some limitations. First, the analysis included AEs rated as grade≥3 with an incidence of≥3%. However, the results of the one-way sensitivity analyses demonstrated the economic results were not sensitive to AEs-related parameters. Second, several key parameters in the analysis, such as utility scores, cost of AEs-related treatments and cost of orchidectomy, were derived from the literature. The utility scores specific to the Chinese population were unavailable on the basis of a comprehensive literature search. Therefore, the utility in our analysis were alternatively derived from previous studies, which may influence our model results. Nevertheless, the impact of variation in the inputs on the model outputs was evaluated in one-way sensitivity analysis. Third, the analysis was performed based on the CHART trial, in which available OS and PFS data are immature, and the median PFS and OS were not reached. Therefore, we obtained the PFS and OS data of the patients using parameter distribution fitting. Although extrapolation could obtain relevant data outside the follow-up period of the CHART trial, this would increase model uncertainty. However, we performed a comparative analysis based on the AIC and BIC to select the best-fitting distribution and a sensitivity analysis to verify the robustness of the model results. In this study, the second-generation drug rezvilutamide was only compared with the first-generation drug. In the future, cost-effectiveness analysis based on meta-analysis or real-world studies can be conducted to evaluate the economics of various antiandrogens in treatment of mHSPC.
Conclusion
In this study, we evaluated the pharmacoeconomic profile of rezvilutamide combined with ADT as a first-line treatment for patients with mHSPC from the perspective of the Chinese healthcare system. After national drug negotiations, rezvilutamide plus ADT has become a more cost-effective treatment option than bicalutamide plus ADT.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
HD and SL contributed equally.
Contributors HD, SL and LF were involved in study conceptualisation and study design. XX, WXu and CH completed the data collection and analysis. SL wrote the initial draft. HD, LF, ZZ and WXin reviewed and edited the draft. LF is responsible for the overall content as the guarantor. All authors approved the final manuscript.
Funding This work was supported by Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province (2020E10021).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.