Article Text
Abstract
Objectives This study aims to investigate the diagnostic value of heparin-binding protein (HBP) in sepsis and develop a sepsis diagnostic model incorporating HBP with key biomarkers and disease-related scores for rapid, and accurate diagnosis of sepsis in the intensive care unit (ICU).
Design Clinical retrospective cross-sectional study.
Setting A comprehensive teaching tertiary hospital in China.
Participants Adult patients (aged ≥18 years) who underwent HBP testing or whose blood samples were collected when admitted to the ICU.
Main outcome measures HBP, C reactive protein (CRP), procalcitonin (PCT), white blood cell count (WBC), interleukin-6 (IL-6), lactate (LAC), Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) score were recorded.
Results Between March 2019 and December 2021, 326 patients were enrolled in this study. The patients were categorised into a non-infection group (control group), infection group, sepsis group and septic shock group based on the final diagnosis. The HBP levels in the sepsis group and septic shock group were 45.7 and 69.0 ng/mL, respectively, which were significantly higher than those in the control group (18.0 ng/mL) and infection group (24.0 ng/mL) (p<0.001). The area under the curve (AUC) value of HBP for diagnosing sepsis was 0.733, which was lower than those corresponding to PCT, CRP and SOFA but higher than those of IL-6, LAC and APACHE II. Multivariate logistic regression analysis identified HBP, PCT, CRP, IL-6 and SOFA as valuable indicators for diagnosing sepsis. A sepsis diagnostic model was constructed based on these indicators, with an AUC of 0.901, a sensitivity of 79.7% and a specificity of 86.9%.
Conclusions HBP could serve as a biomarker for the diagnosis of sepsis in the ICU. Compared with single indicators, the sepsis diagnostic model constructed using HBP, PCT, CRP, IL-6 and SOFA further enhanced the diagnostic performance of sepsis.
- INTENSIVE & CRITICAL CARE
- Infection control
- Adult intensive & critical care
Data availability statement
Data are available on reasonable request. Date are available on reasonable request
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study included a highly heterogeneous population, making it highly applicable to patients with sepsis in the intensive care unit (ICU).
Moreover, most of the biomarkers included in this diagnostic model are widely used in clinical practice, making them easily obtainable, highly reproducible and operationally feasible.
This was an ICU single-centre retrospective study, and the results might be inapplicable to sepsis patients in other settings.
The Sequential Organ Failure Assessment scores in the study were absolute values automatically obtained by the electronic scoring system rather than the delta values.
Its design dose not allow for the determination of causal relationships.
Background
Sepsis is a life-threatening organ dysfunction caused by dysregulated host response to infection. Sepsis, when accompanied by severe circulatory impairment and cellular metabolic disorders, is referred to as septic shock and is the leading cause of death in patients with sepsis.1 With the ageing population and increase in immunocompromised hosts, the incidence of sepsis has recently been rising. The Global Burden of Sepsis study published in 2020 reported 48.9 million cases of sepsis worldwide in 2017, with 11 million deaths attributed to sepsis, accounting for 19.7% of the global deaths.2 Another domestic study showed that the incidence of sepsis in the intensive care unit (ICU) was 20.6%, with a 90-day mortality rate of 35.5%, and the mortality rate for septic shock was as high as 50% or more.3 Im et al demonstrated that the mortality rate of septic shock is correlated with hypotension and the delayed use of antibiotics.4 Another study indicated that early fluid resuscitation is closely linked to the prognosis of patients with sepsis.5 Therefore, early diagnosis and timely and appropriate treatment are crucial for sepsis management.
Early diagnosis and identification of sepsis require a comprehensive approach based on the patient’s clinical symptoms, conventional cultures, biomarkers and disease-specific scoring systems. However, the clinical symptoms and signs of sepsis are often non-specific, and conventional pathogen cultures are relatively delayed.6 Therefore, the early diagnosis of sepsis in the ICU mainly relies on biomarkers and disease-specific scoring systems. Currently, there are over 200 sepsis-related biomarkers have been reported in the literature, among which heparin-binding protein (HBP) is a novel biomarker.7 HBP is a serine protease-like protein secreted by neutrophils after infection that has functions such as altering endothelial cell permeability, antimicrobial activity, chemotaxis and regulation of cell apoptosis.8 It has been identified as an early diagnostic indicator for severe sepsis/septic shock in Chinese Guidelines for the Management of Severe Sepsis/Septic Shock (2014)9 and Chinese Expert Consensus on Early Prevention and Interruption of Sepsis in Emergency Medicine (2020).10 In addition, an increasing number of studies have recently provided evidence regarding the use of HBP for diagnosing sepsis. The results demonstrate that HBP could be used for sepsis diagnosis and severity monitoring.8 11–14 On the other hand, a few studies have indicated elevated levels of HBP irrespective of infectious aetiology and no correlation with severity and outcome.15 Furthermore, differences and inconsistencies have been noted among various studies regarding the diagnostic performance of HBP in sepsis.16 17 Therefore, it remains controversial to use HBP for the early diagnosis of sepsis. This study aimed to analyse the diagnostic value of HBP in sepsis and develop a sepsis diagnostic model combining HBP with multiple biomarkers and disease-specific scoring systems retrospectively to facilitate the identification and diagnosis of sepsis in the ICU.
Methods
Study population
This study included 2080 patients who were admitted to the ICU of the First Affiliated Hospital of Sun Yat-sen University, China, from March 2019 to December 2021. Strict inclusion and exclusion criteria were adopted for all patients, with the following inclusion criteria: (1) patients who underwent HBP detection or whose blood samples were collected for HBP detection at the time of ICU admission, (2) integrity of the clinical data and (3) age 18 years or older. The exclusion criteria were as follows: (1) patients with neutropenia due to haematological malignancies and (2) patients who underwent immunosuppressive therapy. Patients were categorised into four groups (infection, sepsis, septic shock and control groups) based on the final diagnosis at the time of discharge from the ICU or death, determined by the attending physician. Figure 1 displays the flow diagram of the participants.
The flow diagram of participants. HBP, heparin-binding protein; ICU, intensive care unit.
Measurement of plasma HBP and clinical data collection
The previously collected blood samples were sent to the central laboratory to detect plasma HBP levels. Briefly, the blood samples were centrifuged at 1000 rounds/min for 10 min, and a 100 µL of supernatants was collected for plasma level of HBP determination using an immunofluorescence dry quantitative method (Jet-iStar3000, Hangzhou, Joinstar Biomedical Technology). The procedure strictly followed the instructions provided with the reagent kit, and the quality control was performed well.
General information such as gender, age, underlying diseases, site of infection and pathogens were collected. Laboratory tests, such as HBP, procalcitonin (PCT), white blood cell count (WBC), C reactive protein (CRP), interleukin-6 (IL-6) and blood lactate (LAC), were measured at the time of ICU admission. Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were calculated within 24 hours of ICU admission. The length of ICU and survival outcomes (3-day improvement rate and 28-day mortality rate) were also recorded for each group of patients.
Statistical methods
For baseline measurement data, the median and IQR were employed to describe the data. If continuous variables followed a normal distribution, one-way analysis of variance was used for intergroup comparisons; otherwise, the Kruskal-Wallis H test was deployed. Percentage calculations were performed for categorical data, and differences between groups were tested using the χ2 test or Fisher’s exact test.
Receiver operating characteristic (ROC) curves were used to assess the diagnostic performance of HBP, PCT, WBC, CRP, IL-6, LAC, APACHE II score and SOFA score for sepsis. The area under the curve (AUC) was calculated. The optimal cut-off values for diagnosing sepsis were determined based on the maximum Youden index, and the corresponding sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated.
To improve the diagnostic performance of sepsis, a multivariate binary logistic regression model was constructed. Random selection of 70% of all patients was used as the training set, whereas the remaining 30% served as the test set to assess the model’s performance. The AUC was calculated for both the training and test sets. The Hosmer-Lemeshow goodness-of-fit test and calibration curve were used to evaluate the model’s goodness-of-fit for both datasets. Decision curves were plotted to evaluate the clinical utility of the regression model. All hypothesis tests were two tailed, with a significance level of p<0.050. Statistical analyses were performed by using R V.4.1.1 and SPSS V.25.0.
Patient and public involvement
This was a retrospective study. No patients or public representatives were involved in setting the research question, nor in the study design, implementation or interpretation.
Results
Characteristics of the patients
Finally, 326 patients were enrolled, including 93 in the control group, 94 in the infection group, 53 in the sepsis group and 86 in the septic shock group (figure 1). Table 1 summarises the baseline characteristics of the patients. The median ages of patients in the control group, infection group, sepsis group and septic shock group were 56, 63, 58 and 64 years, respectively, with statistically significant differences among the groups (p=0.023). No significant differences were noted among the groups in terms of gender, prevalence of hypertension, diabetes, heart disease, malignancy, liver disease or other comorbidities.
Characteristics of the patients
The control group consisted of patients who recovered postoperatively from various surgical procedures, including gastrointestinal, hepatic, vascular, among others. Patients with infection (including the infection, sepsis and septic shock groups) predominantly presented with pulmonary infections (48.9%, 32.1% and 26.7%, respectively) and abdominal infections (33.0%, 56.6% and 73.3%, respectively). Among all enrolled patients, 32 had positive blood cultures, 76 had positive peritoneal drainage fluid cultures and 90 had positive sputum cultures. All patients with sepsis (including the sepsis and septic shock groups) mainly suffered from bacterial infections and received antibiotic treatment. The APACHE II and SOFA scores of the sepsis and septic shock groups were significantly higher than those of the control and infection groups, with statistically significant differences among the four groups (p<0.001). In the prognosis analysis, the 28-day mortality rates for the sepsis and septic shock groups were 11.32% and 32.56%, respectively, which were significantly higher than those for the control and infection groups (3.2% and 9.6%) (table 1).
Levels of HBP and other biomarkers in each group of patients
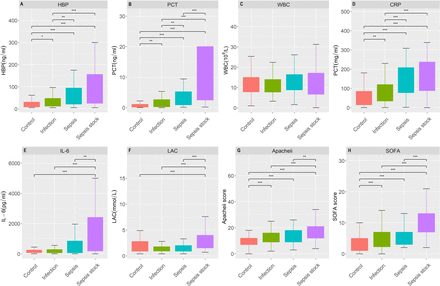
The median (IQR) HBP levels in the control, infection, sepsis and septic shock groups were 18.0 (9.9–32.1), 24.0 (14.1–56.4), 45.7 (24.8–107.9) and 69.0 (33.8–150.9) ng/mL, respectively (p<0.001). HBP was capable of effectively distinguishing between patients with and without infection or sepsis, and its efficacy was superior to that of IL-6, LAC and WBC. However, in distinguishing septic patients with or without shock, HBP was inferior to PCT, IL-6 and LAC. Additionally, no statistically significant differences were noted in WBC among the groups (figure 2).
Comparison of plasma levels of biomarkers among different groups: (A) HBP, (B) PCT, (C) WBC, (D) CRP, (E) IL-6, (F) LAC, (G) APACHE II, (H) SOFA. *p<0.05; **p<0.01; ***p<0.001. APACHE II, Acute Physiology and Chronic Health Evaluation II; CRP, C reactive protein; HBP, heparin-binding protein; LAC, blood lactic acid; PCT, procalcitonin; IL-6, interleukin-6; SOFA, Sequential Organ Failure Assessment, WBC, white blood cell count.
When comparing HBP levels among different infection sites in the infection, sepsis and septic shock groups, statistical differences were observed among the subgroups, except for the multi-infection site (online supplemental table 1). As the severity of infection increased, the APACHE II and SOFA scores gradually increased, showing statistically significant differences. However, no statistical difference was observed between the infection and the sepsis groups (figure 2).
Supplemental material
Analysis of the diagnostic accuracy of different biomarkers for sepsis
HBP demonstrated promising diagnostic performance for the detection of sepsis, with an AUC of 0.733 (95% CI 0.678 to 0.789), which was significantly higher than WBC (AUC 0.541, 95% CI 0.474 to 0.607) and higher than the AUCs of IL-6, LAC and APACHE II scores (0.658, 0.632 and 0.688, respectively), but the difference was not statistically significant. The AUC for HBP was significantly lower than that for PCT (AUC 0.812, 95% CI 0.766 to 0.857). When the HBP cut-off value was set at 35.2 ng/mL, the sensitivity, specificity, PPV and NPV for diagnosing sepsis were 65.5%, 74.9%, 65.9% and 74.5%, respectively (table 2, online supplemental figure 1).
Performance of biomarkers to discriminate sepsis from non-sepsis
Relationship between HBP and other biomarkers
No significant correlation was observed between HBP levels and CRP, PCT, WBC, IL-6, LAC, APACHE II scores and SOFA scores (online supplemental figure 2).
Construction of a sepsis diagnostic model
Based on the training set, variables were selected using univariate logistic regression analysis for patient demographics (such as gender, age, underlying diseases, infection sites and pathogens), infection biomarkers (HBP, PCT, WBC, CRP, IL-6 and LAC), APACHE II scores and SOFA scores. Variables with statistical significance (p<0.05) were included in the multivariate logistic regression model (online supplemental table 2). Statistically significant variables in the univariate analysis were HBP, PCT, CRP, IL-6, LAC, APACHE II and SOFA scores. The final multivariate logistic regression results showed that PCT (OR 1.034, 95% CI 1.009 to 1.060, p=0.009), CRP (OR 1.011, 95% CI 1.006 to 1.016, p<0.001), HBP (OR 1.006, 95% CI 1.000 to 1.012, p=0.041), IL-6 (OR 1.001 95% CI 1.000 to 1.001, p=0.013), SOFA (OR 1.252, 95% CI 1.110 to 1.412, p<0.001) were significantly associated with sepsis diagnosis. The sepsis diagnostic model was constructed based on the results of logistic regression, as illustrated in figure 3.
A nomogram predicting the risk of sepsis for patients. The value of each of variable was given a score on the point scale axis. A total score could be easily calculated by adding each single score and by projecting the total score to the lower total point scale. We were able to estimate the probability of sepsis. CRP, C reactive protein, HBP, heparin-binding protein; PCT, procalcitonin; IL-6, interleukin-6; SOFA, Sequential Organ Failure Assessment.
Validation of the sepsis diagnostic model
To evaluate the predictive performance of the model, the remaining 30% of patients were used as a test set to validate the model. In the training set, the model achieved an AUC of 0.901 (95% CI 0.863 to 0.940). When the Youden index was maximised, the cut-off value was determined to be 0.439, resulting in a sensitivity of 79.4% and a specificity of 86.5%. In the test set population, the model obtained an AUC of 0.913 (95% CI 0.860 to 0.966). Applying the cut-off value obtained from the training set to the test set, the sensitivity and specificity were 80.5% and 87.7%, respectively (online supplemental figure 3). Furthermore, to obtain a more accurate cut-off value, all patients were included in the diagnostic model, resulting in a cut-off value of 0.439. The sensitivity and specificity for diagnosing sepsis with this cut-off value were 79.7% and 86.9%, respectively.
The diagnostic model constructed using the training set exhibited a good predictive performance based on the Hosmer-Lemeshow goodness-of-fit test in the training and test sets (χ2=4.91, p=0.767; χ2=5.12, p=0.745; online supplemental figure 4). Additionally, the decision curve analysis plot demonstrated a high clinical net benefit for the constructed sepsis diagnostic model that surpasses both treat-all and treat-no (online supplemental figure 5).
Discussion
Sepsis is a major cause of mortality in critically ill patients and is associated with high morbidity and mortality rates. Approximately 20%–30% of severely infected patients do not exhibit typical symptoms of organ dysfunction on admission but rapidly progress to sepsis.6 Therefore, early identification of sepsis is crucial for developing appropriate and effective treatment strategies and reducing mortality. Clinicians require specific and sensitive biomarkers for the early diagnosis of sepsis. Currently, WBC, CRP and PCT are commonly used as inflammatory biomarkers in clinical practice.7 However, WBC and CRP are non-specific markers of systemic inflammation and cannot effectively differentiate among bacterial, non-bacterial and sterile inflammation. PCT has a higher specificity for bacterial infections but performs poorly in predicting sepsis-associated organ dysfunction.6 18 In recent years, numerous studies have proven that HBP has good predictive performance for infection, sepsis or organ function assessment, superior to PCT, CRP and other biomarkers.6 8 11 12 19 20
HBP, also known as CAP37, is a protein that is stored in the secretory granules of neutrophils and azurophilic granules. It contains a large number of positively charged amino acid residues that are concentrated on one side of the protein.20 A hydrophobic pocket structure formed by amino acid residues 20–44 exhibits a high affinity for endotoxins.6 Therefore, HBP was initially discovered for its antimicrobial activity. Subsequent studies have confirmed that HBP is a multifunctional innate immune defence molecule that plays a crucial role in the host’s infection and inflammatory responses.6 20 These characteristics make HBP a promising novel infection biomarker. Recent studies have reported that HBP could assist in diagnosing various diseases, such as respiratory and circulatory failure, sepsis, acute kidney injury, acute lung injury, meningitis, urinary tract infections, and skin and soft tissue infections.6 8 11 21–25 However, its clinical use has not yet been widely adopted; accordingly, further clinical research is required to validate its utility.
This study further confirms that HBP is a promising biomarker for sepsis. In this study, HBP levels could effectively differentiate whether patients had an infection and whether infected patients had sepsis. Furthermore, its discriminative value was found to be superior to that of the LAC, IL-6, WBC, SOFA and APACHE II scores. Similar findings have been previously reported.7 11 These results were likely related to the biological characteristics of HBP. It is stored in neutrophil secretory granules and azurophilic granules, and on stimulation by pathogens, it can be rapidly and massively released into the bloodstream, inducing rearrangement of the endothelial cell cytoskeleton, leading to vascular leakage and oedema formation. Additionally, HBP regulates the function of monocytes and macrophages, further amplifying the inflammatory response and enhancing the body’s immune response to infection. Moreover, as neutrophils infiltrated into the tissues, HBP continued to be released, resulting in tissue damage and organ dysfunction.20 26 Consequently, HBP levels were significantly elevated in patients with infection and/or sepsis.
Regarding the diagnostic performance of HBP in sepsis, Linder et al found that the AUC of HBP for predicting sepsis was 0.85, with a sensitivity of 87% and specificity of 95%, which were significantly higher than those of PCT, CRP, WBC, IL-6 and other biomarkers.8 Furthermore, HBP can predict the occurrence of organ dysfunction and circulatory failure at an early stage, providing indications for timely interventions such as fluid resuscitation and antibiotic use, which are indispensable components of sepsis bundle therapy.8 11 27 In addition, the favourable predictive value of HBP was validated in paediatric patients with severe sepsis.28 The emergence of this phenomenon was considered to be linked to the pathological process in which HBP is involved in vascular leakage and organ dysfunction in septic patients, and its release occurred earlier than CRP, PCT and other markers.19 20 26 In this study, the AUC of HBP in predicting sepsis was 0.733, which was not superior to PCT, CRP and SOFA. Previous studies have reported varying diagnostic accuracies of HBP for sepsis at different time points.19 In this study, patients underwent HBP testing on ICU admission or had plasma collected at that time for subsequent HBP assessment. Consequently, HBP levels were measured for all patients at the time of ICU admission. Since a definitive diagnosis of sepsis required a comprehensive evaluation based on subsequent examinations, diagnoses were collected after patient discharge or death. Therefore, the timing of HBP testing or blood sample collection preceded the definitive diagnosis but might not represent the early stage of sepsis. Based on this, HBP did not demonstrate high diagnostic efficiency for the early detection of sepsis in this study. Meta-analyses also revealed that HBP often performed better in diagnosing sepsis in emergency department patients compared with ICU patients.15 16 19 Unlike previous studies, this study involved ICU patients rather than emergency patients. First, the control group in this study consisted of surgical postoperative recovery patients without infection. Additionally, ICU patients have more complex conditions, have more severe organ damage and require life support, such as ventilators, vasopressors and continuous renal replacement therapy. Finally, the patients already received various treatments, such as fluid resuscitation and antibiotics in the emergency room or ward.29–33 In summary, these conditions might have some impact on HBP levels, but this study population was more representative of the actual situation of ICU patients. From another perspective, this phenomenon also reflects the limitations of a single biomarker, as it could not fully reflect the clinical reality and accurately diagnose sepsis in the ICU.
The pathophysiological mechanisms that underlie sepsis are complex. They are involved in different immune states, sites of infection and pathogens. Immune response patterns vary, as do the pathophysiological processes of various biomarkers. During its occurrence and progression, dual factors simultaneously lead to an exaggerated inflammatory response and immune dysfunction. Systemic inflammatory responses and immune suppression do not generally exist as simple independent entities but rather coexist. Therefore, a single biomarker cannot serve as a reliable diagnostic indicator for sepsis.7 10 In this study, we also observed that HBP showed almost no correlation with PCT, CRP, IL-6, LAC, APACHE II and SOFA scores. This suggests that HBP, as a biomarker, could provide unique information for diagnosing sepsis independent of other biomarkers. We hypothesised that establishing a diagnostic model combining HBP with PCT, CRP, IL-6, LAC, APACHE II, SOFA scores and other indicators could be a new approach to the diagnosis of sepsis. Currently, relevant studies have been conducted in this regard,34 35 however, many of the biomarkers mentioned in the above studies have not been widely used in clinical practice, making them less practical. In this study, biomarkers commonly used in clinical settings were included. Based on the ROC analysis of various markers, a sepsis diagnostic model was constructed using multivariable logistic regression. On testing, the sepsis diagnostic model exhibited an AUC of >0.90, indicating its high clinical applicability.
Conclusion
This study confirmed the value of plasma HBP levels in the diagnosis of sepsis in the ICU. It also constructed a sepsis diagnostic model that includes HBP, PCT, CRP, IL-6 and SOFA scores. This model demonstrated a high accuracy and clinical utility, further enhancing its predictive role in sepsis. It has potential clinical diagnostic value for the detection of sepsis in the ICU.
Data availability statement
Data are available on reasonable request. Date are available on reasonable request
Ethics statements
Patient consent for publication
Ethics approval
The protocols were approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University and were conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We appreciate Yanzhe Xia from the department of pharmacy and Kang Liao from the microbiology laboratory for their professional support of this study and their careful interpretation of medication guidance and each specimen’s aetiology.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
LZ, XL, LW and HY are joint first authors.
LZ, XL, LW and HY contributed equally.
Contributors Study concept and design: YL and LZ. Definition of the diagnostic algorithm: YL, JW and XG. Data acquisition and analysis: LZ, XL, ZL and SZ. Data interpretation: LW and HY. Manuscript drafting: LZ, XL, LW, HY and YL. Manuscript revision: all authors.YL is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.