Article Text
Abstract
Objectives This study aims to examine the prevalence of comorbidities in adult patients with psoriasis and compare them with those in control subjects without psoriasis in Tianjin, China.
Design The study is a cross-sectionalanalysis.
Participants The participants were established by identifying all patients (age ≥18 years) who visited hospitals and clinics in Tianjin between 1 January 2016 and 31 October 2019.
Setting The study group consisted of 20 678 adult patients with psoriasis, and a comparison group was created after 1:1 propensity score matching. Logistic regression analyses were conducted to examine the risk of 22 comorbidities for these two groups.
Results Patients with psoriasis had a significantly higher prevalence of 11 comorbidities and a lower prevalence of 2 comorbidities within 12 months of follow-up. Our results also showed that the proportion of psoriatic arthritis might account for approximately 2% of all patients with psoriasis. This psoriatic arthritis group had a higher average age and CCI (Charlson Comorbidity Index) index score (2.27 >1.62, p <0.001) than the non-arthritis group.
Conclusions This study showed that psoriasis in Tianjin is associated with various comorbidities. It also emphasises the importance of clinical treatment in improving therapeutic effects and reducing the burden of psoriasis in China.
- Psoriasis
- Dermatological epidemiology
- Adult dermatology
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study involved a large cohort of participants (n=20 678) for cross-sectional analysis.
To mitigate potential confounding factors, subjects were matched 1:1 based on propensity scores in each group.
ORs were adjusted for confounding variables to assess the independent relationship between medical comorbidities in patients with psoriasis compared with the control group using sensitivity analysis.
The cross-sectional design of this study limited our ability to establish causal inferences regarding psoriasis and comorbidities.
Due to incomplete electronic medical records in the database, the impact of severity and other potential variables on comorbidities could not be observed.
Introduction
Psoriasis is an immune-mediated condition that affects not only the skin but also extracutaneous systems. The disease burden is likely greater than reported due to the associated comorbidities,1 including cardiometabolic, gastrointestinal, renal, malignancy, infection, mental and ocular diseases, and PsA (PsA).2 3 The abnormal immune response that causes psoriasis leads to systemic inflammation and a higher prevalence of comorbidities compared with the general population.4 Understanding these comorbidities is crucial for better disease management and reducing the burden on individuals and society.5
According to the ‘Global Burden of Disease study’ in 2019, the prevalence of psoriasis in China is estimated to be 0.56%,6 suggesting based on the estimated population of China in 2023, which is approximately 1.4 billion people, a prevalence rate of 0.56% would correspond to over 7 million individuals with psoriasis in the country. Despite China having the largest population and the most prevalent cases of psoriasis,7 epidemiological studies on psoriasis prevalence and comorbidity risk are rare. Available data mainly come from the USA and Europe,1 but baseline disease characteristics and comorbidity frequencies may differ between geographical regions,8 including China. The WHO has recently released a report to bring attention to the public health impact of psoriasis. The report emphasises that obtaining quality data on the epidemiology of psoriasis is a crucial area of research globally better to understand the size and distribution of the problem. Such data are essential for disease control and appropriate healthcare planning.
Hence, to address the gap in epidemiological data on the prevalence of psoriasis comorbidities in China, we conducted a retrospective study to determine the prevalence of various comorbidities in adult patients with psoriasis. Our study also compared these prevalences with those of control subjects without psoriasis in Tianjin.
Methods
Study design
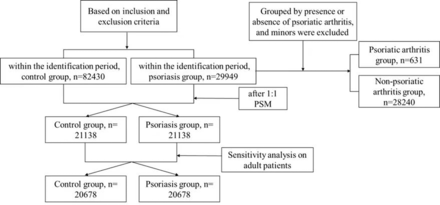
This study used data from the Inspur Health Database in Tianjin to examine the prevalence of comorbidities in adult patients with psoriasis compared with a control group. The study and control groups were established by identifying all patients (age≥18 years) who visited hospitals and clinics in Tianjin between 1 January 2016 and 31 October 2019. Patients with two or more psoriasis-related diagnoses were selected as the study group, while those who had never received a psoriasis diagnosis were classified as the control group. The incidence date for the psoriasis cohort was defined as the date of the first psoriasis diagnosis, while the index date for the control group was defined as the first hospital visit during the recognition period. After excluding individuals with abnormal age, visiting frequency or missing data, 20 678 subjects were included in each group following 1:1 propensity score matching (PSM). Follow-up continued until the end of the study period on 31 October 2020. The baseline period encompassed data from at least 12 months prior to the incidence or index date, while the follow-up period comprised data from at least months after the incidence or index date. The flow of participants through the study is illustrated in a Consolidated Standards of Reporting Trials diagram in figure 1.
A Consolidated Standards of Reporting Trials diagram to show the study process.
Outcome
In this study, we focused on several health outcomes of interest, including hypertension, dyslipidaemia, diabetes, hyperuricaemia, obesity, metabolic syndrome, myocardial infarction, coronary atherosclerotic heart disease, cerebrovascular disease, peripheral vascular disease, inflammatory bowel diseases (IBD), non-alcoholic fatty liver disease (NAFLD), chronic kidney disease (CKD), infection, thyroid disease (including autoimmune thyroid disease, hypothyroidism, hyperthyroidism, thyroiditis and thyroid cancer), psychiatric diseases, rheumatoid arthritis, arthritis, AIDS, lymphoma and malignancy. The ICD-10(International Classification of Dieases-10) diagnostic codes chosen for each outcome were based on medical judgement.
Statistical analysis
Categorical variables were reported as frequency and percentage, while continuous variables were summarised using the maximum, minimum, mean, SD, median and IQR. Due to the non-normal distribution of constant data, the Wilcoxon rank sum test or Kruskal-Wallis rank sum test was used to compare group differences, as appropriate. The χ2 or Fisher’s exact test was used to compare categorical variables between groups. A p value of less than 0.05 was considered statistically significant.
To address potential confounding factors, a PSM analysis was conducted. A logistic regression model was developed, including age, sex, hospital visited and type of medical insurance as covariates to generate scores, with age as the primary matching variable. One-to-one nearest-neighbour matching was performed using a calliper width of 0.05. A standardised mean difference value of less than 0.2 was considered a good match.
Sensitivity analysis
To assess the robustness of our results, we constructed a logistic regression model and a corresponding forest plot to investigate any differences in the prevalence of the comorbidities of interest between the study and control groups over the follow-up period. The dependent variable was whether individuals developed the relevant outcomes during this period, while the independent variable was the group (psoriasis group vs control group), as well as the presence of the target comorbidities at baseline. We obtained the OR and its 95% CI, with statistical significance defined as a two-sided p value of less than 0.05.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
The distribution of baseline characteristics between psoriasis and non-psoriasis groups before and after PSM is presented in table 1. Among the 41 296 sampled patients, the mean ±SD age was 45.37 ±16.4 years, with 47% and 44.7% being female in the control and psoriasis groups, respectively. No significant differences were observed between these two groups based on gender, age, type of health insurance and hospital grade.
Differences in baseline characteristics between patients with psoriasis and controls before and after propensity score matching (PSM)
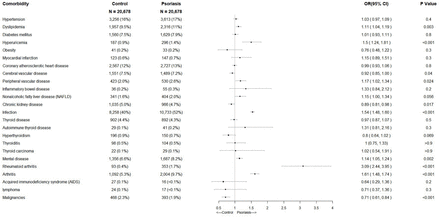
The prevalence of comorbidities in patients with and without psoriasis is shown in table 2 and online supplemental STable 1 with the primary analysis methods of Pearson χ2 independence test and Fisher’s exact test. The results indicate that patients with psoriasis had a significantly higher prevalence of 11 comorbidities, including hypertension, dyslipidaemia, hyperuricaemia, coronary atherosclerotic heart disease, peripheral vascular disease, IBD, NAFLD, infection, psychiatric disorders, rheumatoid arthritis and arthritis. On the other hand, patients with psoriasis had a considerably lower prevalence of two comorbidities, including hyperthyroidism and malignant tumour, within 12 months of the follow-up period. The sensitivity analysis showed that patients with psoriasis had significantly higher probability of developing dyslipidaemia (OR (95% CI) 1.11 (1.04 to 1.19), p=0.003), hyperuricaemia (OR (95% CI) 1.50 (1.34 to 1.81), p<0.001), peripheral vascular disease (OR (95% CI) 1.17 (1.02 to 1.34), p=0.024), infection (OR (95% CI) 1.54 (1.48 to 1.60), p<0.001), psychiatric disorders (OR (95% CI) 1.14 (1.05 to 1.74), p<0.001), rheumatoid arthritis (OR (95% CI) 3.09 (2.44 to 3.95), p<0.001) and arthritis (OR (95% CI) 1.61 (1.48 to 1.74), p<0.001), while they had significantly lower probability of developing malignancy (OR (95% CI) 0.71 (0.61 to 0.84), p<0.001), cerebrovascular disease (OR (95% CI) 0.92 (0.85 to 1.00), p=0.04) and CKD (OR (95% CI) 0.89 (0.81 to 0.98), p=0.017), compared with the control group during the 12-month follow-up period. These results are presented in figure 2.
Supplemental material
Prevalence of medical comorbidities in patients with psoriasis versus comparison of two groups in baseline and 12-month follow-up period
Adjusted ORs of medical comorbidities in patients with psoriasis versus comparison group by sensitivity analysis (OR >1 indicates that patients with psoriasis have greater incidence).
We further analysed patients with psoriasis into those with or without PsA. There was 2% PsA in all of patients with psoriasis. Meanwhile, the average age was higher in PsA group than in the non-arthritis group (p <0.001). No gender difference was observed (p=0.4). Moreover, patients with PsA were more likely to have comorbidities such as hypertension, dyslipidaemia, diabetes, hyperuricaemia, coronary atherosclerotic heart disease, peripheral vascular disease, NAFLD, CKD and rheumatoid arthritis. These results are shown in table 3. Additionally, we compared the CCI index in two groups during the 12-month follow-up period using the Wilcoxon rank sum test. The PsA group had a distinct higher CCI index score (2.27 >1.62, p <0.001).
Comorbidities in patients with psoriatic arthritis versus without psoriatic arthritis group
Discussion
It is widely recognised that patients with psoriasis usually suffer a heavy economic and mental burden. It affects not only the skin level but also multiple systems and organs. Numerous epidemiological data on psoriasis have been collected from European countries, the UK and the USA1 that addressed comorbidities are pretty common in patients with psoriasis; however, there is little few information on Asian populations. This study aimed to proceed with a large retrospective study based on the Inspur Tianjin Health Database to assess the comorbidities in patients with psoriasis in Tianjin and provide more insights on psoriasis in the Han nationality. Due to the COVID-19 outbreak, the time for us to supplement and optimise data and analysis results has been extended, and it was not until recently that all of it was completed.
Our current study found that the comorbidity with the highest prevalence among patients with psoriasis was infection, with a significantly higher prevalence than that of the control group (52% >40%, p <0.001). Patients with psoriasis are at an increased risk of infection, which may be due to treatment with immunomodulatory or immunosuppressive drugs.9 Vaccinations may prevent specific infections, but they can also trigger and exacerbate psoriasis, as studies have shown in relation to influenza vaccination.10 There have also been reports of psoriatic disease exacerbation triggered by COVID-19 mRNA vaccination, with the mechanism similar to that of other vaccines in that vaccination induces interleukin (IL)-6, which stimulates Th17 cells to produce IL-22, a significant contributor to keratinocyte proliferation in psoriasis.11 Increasing epidemiological studies have recently shown a close correlation between psoriasis and metabolic syndrome and cardiovascular factors. Our findings are consistent with these results, with the most prevalent comorbidities being hypertension (17%), hyperlipidaemia (11%), diabetes mellitus (7.9%) and coronary heart disease (13%). The origin of the association between psoriasis and cardiovascular factors remains uncertain. However, it is plausible to consider that chronic low-grade systemic inflammation and concomitant proinflammatory cytokine activity may contribute to vascular damage and increased cardiovascular risk. The exact role of the IL-23/IL-17 axis in atherosclerosis is still debated, but studies have shown an accumulation of IL-17-producing cells and elevated levels of IL-17A in atherosclerotic lesions.12 13 Additionally, besides individual genetic predisposition, changing metabolites may elucidate the underlying mechanism linking psoriasis and cardiovascular diseases.14 Although there was no significant difference between the two groups, the prevalence of diabetes mellitus was slightly higher in the psoriasis group than in the control group. The two diseases share a common genetic aetiology and numerous pathophysiological mechanisms connected to an upregulation of proinflammatory cytokines, adipokines, receptors for peptide-1-glucagon-like and incretin.15 It is noteworthy that the emergence of IL-17/23 inhibitory monoclonal antibodies has revolutionised the therapeutic approach to psoriasis, with increasing scientific evidence supporting their use as first-line therapy in patients with cardiovascular comorbidities and metabolic syndrome.16 Psoriasis is associated with various negative impacts on mental health, including increased risks of anxiety, depression, low self-esteem, alexithymia, stress, self-harm and suicidality.17 Patients with psoriasis experience greater mental health comorbidity burdens18 19; a recent large case-control study from Denmark evaluated the occurrence of mental health disorders by reviewing patient records and found that mental health disorders were observed in 3.1% of patients with psoriasis compared with 2.2% of controls.20 This finding is consistent with our results showing that the prevalence of psychiatric disorders was significantly higher in patients with psoriasis (8.2% vs 6.6%, p <0.05). Some research focuses on the impact of psychiatric complications during psoriasis treatment.21 Dermatologists need to screen patients with psoriasis for psychiatric comorbidities and provide appropriate mental health support. IBD has caused attention among comorbidities of psoriasis, with a prevalence about 0.3%.22 There is growing evidence that they could interact with each other. Our results confirm that the prevalence of psoriasis in our study group is significantly higher than that of the control group (0.3% >0.2%, p =0.046), which is consistent with other studies. There are many overlaps in pathophysiological mechanisms which include extracellular tumour necrosis factor, IL-23, IL-17 signalling pathways, intracellular JAK-STAT pathway, cAMP signalling pathway and ROR-γ T/Th17 axis between the two conditions; consequently, drugs targeting these common pathways have become a hot topic in treating these two comorbidities.23 24 NAFLD is now regarded as the hepatic manifestation of metabolic syndrome. The relationship between psoriasis and NAFLD was independent of other hepatic risk factors, such as potentially hepatotoxic antipsoriatic therapy and alcohol consumption.25 Our results confirm that the prevalence of NAFLD in patients with psoriasis is significantly higher than in controls (2.0% >1.6%, p =0.020). NAFLD might actively contribute to the severity of psoriasis through the release of pathogenic mediators from the inflamed liver26; the systemic release of proinflammatory/proatherogenic mediators from the steatotic liver is also one of the underlying mechanisms by which NAFLD may contribute to accelerated atherogenesis.27 It is worth noting that the presence of NAFLD should be taken into consideration when choosing therapy, as some antipsoriatic drugs are potentially hepatotoxic.28
Our study found a lower incidence of malignant tumours compared with the control group (1.9% < 2.3%, p=0.01), which contrasts with some other studies. Chronic inflammation and impaired immune surveillance have been suggested to be linked to an increased risk of cancer.29 A 2013 meta-analysis reported an increased risk of solid cancers in the upper aerodigestive tract including the oesophagus, lung, liver and pancreas. However, after adjusting for cigarette smoking and alcohol abuse, they were unable to replicate the increased risk of lung, oesophagus or urinary tract cancer, suggesting an associated rather than an independent risk in patients with psoriasis. The risk of squamous cell carcinoma is increased in patients treated with psoralen combined with ultraviolet A, which has been accepted by most studies but was not tested in our study.30 More prospective studies are needed to investigate this controversial issue.
PsA can lead to joint destruction, deformity, reduced functional status and an increased risk of death.31 Undiagnosed PsA is common among patients with psoriasis, ranging from 10% to 40% in previous studies.32 In our study, the rate of psoriasis combined with arthritis was 9.7%; only 2% of patients had PsA, falling within the upper range of the 1.3%–34.7% reported by the WHO global report on psoriasis. This may be attributed to genetic differences and diagnostic criteria or physicians failing to examine joint symptoms in patients without active complaints of joint pain in the dermatology outpatient clinic. PsA usually develops 8–10 years after the onset of psoriasis, and a delay in diagnosis of 6 months can lead to peripheral joint damage and functional disability.33 Our study found that the PsA group patients were older and had a higher CCL index compared with patients without arthritis, indicating longer disease duration and worse prognosis. Our findings are consistent with prior reports which found a higher prevalence of cardiovascular disease and associated risk factors, such as diabetes and other chronic diseases, in patients with PsA compared with patients with psoriasis in the community.34 Multiple comorbidities in a single patient can make selecting therapeutic agents challenging due to safety concerns and create challenges in assessing the functional impact of PsA. Therefore, early diagnosis and treatment are crucial to improve patient outcomes. Screening for PsA soon after diagnosing psoriasis may lead to earlier identification, allowing for earlier treatment and prevention of joint damage and disability.
This study confirms that common complications may also occur in patients with psoriasis, which may affect their health and social interactions. It filled a gap in the epidemiological data of psoriasis comorbidities in China, providing insights on the understanding of the condition’s causes, quality of life, healthcare trends and research priorities. Additionally, the study highlights the importance of identifying environmental factors that could influence psoriasis and its comorbidities, which can inform policy decisions and quantify the financial burden to society. However, the study has some limitations due to the retrospective studies based on health databases. First, the temporal trajectory of psoriasis could not be fully determined, requiring a more extensive dataset to identify the optimal time to intervene and reduce the risk of comorbidities. Second, there may be potential selection bias since the control population consisted only of patients who visited the hospital, who may themselves have comorbidities or high-risk factors for these conditions. Third, the study was based on a regional database rather than a national medical insurance database, and patient mobility may have caused some patients with psoriasis from other regions not to seek treatment for other diseases in Tianjin, which may have impacted the data. Fourth, the project commenced in 2021, a time when Chinese hospitals lacked comprehensive electronic medical record systems for patient visits. Consequently, we were unable to evaluate the influence of factors such as psoriasis severity, smoking history and alcohol consumption on comorbidities. Additionally, the predetermined 1-year follow-up period was relatively brief. As a result, the current findings do not furnish adequate evidence to establish a relationship between psoriasis therapies and comorbidities. It is important to emphasise that the sensitivity analysis results are partially consistent with the main analysis method, which suggests that the results obtained through the main analysis are more reliable. Inconsistent diseases are also given priority in the main analysis, but the level of evidence may be lower.
Further exploration is needed to determine whether the prevalence of diseases such as hyperthyroidism, CKD and cerebrovascular disease decreases in patients with psoriasis. Overall, this study provides valuable insights into the burden of comorbidities in patients with psoriasis in China and highlights the need for early diagnosis and management of these comorbidities to improve patient outcomes. It will be important for future studies to address these limitations and to use a more representative sample to validate these findings. Nonetheless, this study highlights the need for a comprehensive approach to the management of patients with psoriasis.
Conclusion
This study represents the most comprehensive and extensive cross-sectional investigation of psoriasis comorbidities conducted in certain regions of China. The results reveal a high prevalence of comorbidities, including cardiovascular diseases, metabolic diseases, infections, psychiatric disorders and PsA, which is consistent with findings from other countries. More attention should be given to early screening for patients with PsA because of its high prevalence and poor prognosis. These findings highlight the need for dermatologists to be aware of the high prevalence of these comorbidities in Chinese patients with psoriasis, to provide optimal care. In conclusion, the high prevalence of comorbidities emphasises the importance of better treatment options and therapeutic management to improve clinical outcomes and reduce the burden of psoriasis in China.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and received approval from the Medical Ethics Committee of Tianjin Medical University General Hospital (approval number: IRB2021-WZ-171). Participants gave informed consent to participate in the study before taking part.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors YZ and YG led the writing, reviewing and editing of the manuscript. KZ, LF, JM, YL, QZhou and QZhao contributed to the conceptualisation, reviewing and editing of the manuscript. HW and SH led the conceptualisation and administration of this project and also contributed to reviewing and editing the manuscript. HW is the guarantor.
Funding This work was supported by Scientific Research Projects of Tianjin Key Medical Discipline (Specialty) Construction Project (No: TJYXZDXK-057B).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.