Article Text
Abstract
Objectives To assess whether patients from minority ethnic groups have different perceptions about the quality-of-life outcomes that matter most to them.
Design Cross-sectional observational study.
Setting High volume eye centres serving the most ethnically diverse region in the UK, recruiting from July 2021 to February 2022.
Participants 511 patients with primary open-angle glaucoma and the predisease state of ocular hypertension.
Main outcome measures The main outcome was participants’ self-reported priorities for health outcomes.
Results Participants fell into one of four clusters with differing priorities for health outcomes, namely: (1) vision, (2) drop freedom, (3) intraocular pressure and (4) one-time treatment. Ethnicity was the strongest determinant of cluster membership after adjusting for potential confounders. Compared with white patients prioritising vision alone, the OR for black/black British patients was 7.31 (95% CI 3.43 to 15.57, p<0.001) for prioritising drop freedom; 5.95 (2.91 to 12.16, p<0.001) for intraocular pressure; and 2.99 (1.44 to 6.18, p=0.003) for one-time treatment. For Asian/Asian British patients, the OR was 3.17 (1.12 to 8.96, p=0.030) for prioritising intraocular pressure as highly as vision. Other ethnic minority groups also had higher ORs for prioritising health outcomes other than vision alone: 4.50 (1.03 to 19.63, p=0.045) for drop freedom and 5.37 (1.47 to 19.60, p=0.011) for intraocular pressure.
Conclusions Ethnicity is strongly associated with differing perceptions about the health outcomes that matter. An individualised and ethnically inclusive approach is needed when selecting and evaluating treatments in clinical and research settings.
- ophthalmology
- glaucoma
- health equity
- quality of life
Data availability statement
Data are available on reasonable request. The data that support the findings of this study are not openly available to avoid compromising individual privacy. However, anonymised data are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
To ensure that our findings are not unfairly biased against ethnic minority groups, we recruited from the most ethnically diverse region in the UK so that nearly 50% of the participants were from ethnic minority groups.
We have adjusted for the confounding effect of socioeconomic status by including individual-level data on education, income and occupation in our logistic model.
We assessed covariates captured from routinely collected medical record data across the whole range of disease severity and treatment history, thereby maximising the generalisability of our findings.
Preference elicitation using Best–Worst Scaling may involve a cognitive burden for respondents.
It may have been difficult for some participants to choose between health priorities that were all considered to be important.
Introduction
Important differences in health outcomes by ethnic group are well recognised. In the USA, all-cause mortality is substantially higher for the black ethnic group compared with the white ethnic group across the life span.1 2 In England and Wales, mortality from ischaemic heart disease is highest in the Bangladeshi, Pakistani and Indian groups.3 Apart from mortality, wide inequalities in self-reported health-related quality of life (QoL) have been identified between different ethnic groups in the UK. The negative effect on QoL among Bangladeshi, Pakistani, Arab and Gypsy or Irish Traveller ethnic groups is similar to or greater than the impact of being 20 years older in the whole population.4 To address ethnic inequalities in health outcomes, it is important to understand the underlying reasons.5 6
Differences in socioeconomic position, access to care and healthcare experience may be partly accountable.7–9 But even after controlling for social and economic disadvantage, differences in health still exist.10–12 Beyond social determinants, possible explanations for disparities in health outcomes by ethnic group include differential susceptibility to disease and differential responses to treatment. These issues are exemplified by glaucoma, a chronic disease that is the leading cause of irreversible blindness and accounts for approximately 80% of blindness globally.13 14 For black patients compared with white patients, glaucoma is more prevalent, develops 10 years earlier on average and is 15 times more likely to cause visual impairment.15–17 The outcomes of medical and surgical treatment for glaucoma are worse for black than white populations.18 19 However, the idea that health risks are inevitably associated with particular ethnic groups or genetic profiles is being challenged. Conveying race as a disease risk factor without context may be a form of structural racism that perpetuates stereotypes of some groups as more diseased than others.6 20 21 Diagnostic and treatment algorithms or guidelines that inappropriately take ethnicity into consideration may lead to unsuitable treatment, exacerbating disparities.6 20 21 Thus, there is a pressing need to better understand why health outcomes are worse in minority ethnic groups.4
Minority ethnic and other under-represented groups affirm that their needs and preferences should be used to improve healthcare delivery and outcomes.22 Moreover, they find providers and researchers to be unresponsive to their medical needs regarding treatment options. It has been suggested that ethnic groups perceive aspects of their QoL differently because they respond differently to instruments that measure it.23 Yet, there is scant evidence about whether individual patients have different priorities for health outcomes and, if so, how ethnicity may influence those differing priorities. To address this poverty of health outcome data and help promote equitable healthcare in underserved populations,24 we now examine this question directly.
Methods
Study population
Patients who were under treatment at Moorfields Eye Hospital and St George’s University Hospital, UK were identified and screened from July 2021 to February 2022. These National Health Service (NHS) centres serve the most racially diverse population in the UK, receiving referrals from both community practitioners and secondary care.25 All participants had to have been diagnosed with open-angle glaucoma or the closely related condition of ocular hypertension and to have already experienced treatment (eye drops, laser or surgery) to lower intraocular pressure. Patients with other ophthalmic pathology were excluded. Participants were required to be able to understand, read and speak English without translation. After written informed consent was obtained, participants completed the discrete choice experiment (DCE). Self-reported sociodemographic data (gender, ethnicity, income, education, employment status, marital status) were collected by questionnaire. Ethnicity was classified according to Office for National Statistics (ONS) categories used in the NHS.25–27 Although race and ethnicity can be defined separately, they are often used interchangeably. The terms race and ethnicity were used in this article in line with current recommendations.28 29 Education, employment and income were classified according to UK Biobank criteria.30
Clinical evaluation
To maintain the real-world nature of the data, clinical parameters such as intraocular pressure, visual fields (VFs), visual acuity and medication were obtained during standard clinical care episodes. For the analysis of clinical parameters (listed in table 1), we set identical timeframes for each patient over the 60 months leading up to the date of recruitment. VF mean deviation (MD) was extracted from the Humphrey Field Analyzer 24-2 Swedish Interactive Threshold Algorithm (Carl Zeiss Meditec, Dublin, California, USA).
Sociodemographic and clinical characteristics of patients included in the study
The mean MD from the two most recent VFs within an 18-month time window was calculated to estimate disease severity in each eye at the time of recruitment, and thereby define better and worse eye for each participant. We chose to take a mean from two VFs to reduce noise in the data owing to expected variability in test performance. However, we limited the analysis window to 18 months to minimise error introduced through true progression of disease.
To estimate disease progression for each eye, the rate of change of MD was calculated by linear regression on all VF conducted during the 60-month timeframe.
Discrete choice experiment (DCE)
To elicit individual-level health outcome priorities from participants, we conducted a DCE using Best–Worst Scaling (BWS), a preference elicitation method introduced by Flynn and Louviere.31 32
We adopted a ‘case 1’ BWS design in which multiple small subsets of outcomes are shown to patients, who are asked to choose the most important (best) and the least important (worst) of the outcomes in each presented subset.33 Ranking small subsets of outcomes in a BWS design is cognitively straightforward and produces more robust results than being required to consider all outcomes simultaneously. Moreover, BWS delivers a score showing the relative importance of outcomes, not just a rank order. The method only requires an assumption of ordinality.
Our previous work identified outcomes related to disease and treatment that were important to patients who have glaucoma.34 These outcomes were control of intraocular pressure (eye pressure), maintaining vision, being independent, having a one-time treatment, drop freedom (freedom from using eye drops to control eye pressure) and having a treatment that does not change. We decided to consider one additional outcome related to treatment, namely avoiding side effects of eye drops, because this outcome was coded most frequently across the previous study and was thus potentially important. To ensure all seven outcomes were presented an equal number of times, we used a balanced incomplete block design35 to generate three outcomes in each of seven sets.
Pilot testing of the DCE with patients was performed to ensure that the instructions were clear. Both BWS and sociodemographic data were collected in person from participants in the hospital setting immediately following recruitment. To mitigate the risk of data entry errors, responses were keyed directly into a secure web-based platform and managed electronically.
Sample size
BWS measures are derived from multinomial frequency counts. Thus, the sample size for this study was calculated based on a multinomial distribution.36 37 Under the assumption of the worst possible case in which 3 of the outcomes are selected equally one-third of the time and all other outcomes are never selected, 510 patients were required to ensure that at least 95% of all estimated probabilities of a category being selected are within 0.05 of their true probability.
Statistical analysis
Discrete choice experiment
For each participant, we calculated the BWS Score, defined as the number of times an outcome was chosen as the most important (best) minus the number of times an outcome was chosen as the least important (worst) among the presented outcomes.35 To confirm whether the BWS tasks had been completed appropriately, we checked the relationship between the aggregated most and least counts across the seven outcomes. To assess the consistency of participants’ choices, the distribution of individual-level variance was assessed.35 In addition, the distribution of individual scores for each outcome was checked. Analyses were performed using R software (V.4.2.1) and IBM SPSS (V.20).
Cluster analysis
To identify participants whose priorities are similar, we applied cluster analysis to participants’ BWS scores. Cluster analysis is a technique to classify participants into groups (clusters) that are homogenous within themselves and heterogeneous between each other.38 39 A two-step cluster analysis was chosen, as it creates clusters based on categorical and continuous variables and identifies the optimum number of clusters. Satisfactory cluster formation was verified using logistic regression with BWS scores as covariates.
Multivariate regression model
The association of cluster membership with sociodemographic variables and clinical characteristics was analysed using a multinomial logistic regression model. To control for social and economic disadvantage, we included education, employment and income as individual-level measures of deprivation. Relevant variables related to disease severity and treatment history were selected using clinical judgement then refined using Pearson correlation matrices and variance inflation factors to avoid multicollinearity. We used the MICE package in R to impute missing data to minimise potential bias and conducted a sensitivity analysis using complete case records to verify the result of the primary analysis.
Patient and public involvement
No patients were directly involved in setting the research question, outcome measures, study design or implementation. No patients were involved in the interpretation or writing up of results. Researchers involved in the study will disseminate the results to patients and the public through relevant websites and conferences at the national level.
Results
Patients’ characteristics
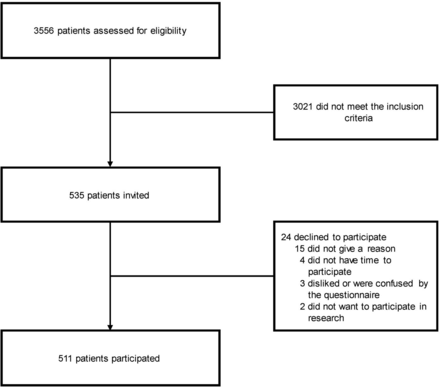
511 patients agreed to participate, representing over 95% of those eligible and invited to take part (figure 1). Approximately half of the participants were white (273/511 (53.4%)) and 55.4% (283/511) were male. Overall, participants had a mean (±SD) age of 67.6 (12.4) years, with a mean duration from diagnosis (living with glaucoma) of 8.5 (7.3) years. Patients had a wide range of disease severities and treatment histories. Sociodemographic and clinical characteristics of the participants are reported in table 1.
Flowchart. Number of individuals at each stage of the study.
Discrete choice experiment (DCE)
All participants completed the DCE, with no missing data. To check the performance of the DCE, we conducted several tests. As shown in figure 2A, aggregate best and worst counts were inversely related, confirming that the DCE was performing appropriately across all participants. Individuals’ response consistencies were also checked. Most participants exhibited high score variance (figure 2B), confirming that most participants gave consistent responses.
(A) Graph of most (x) versus 1/(least) (y) for aggregate Best–Worst Scaling (BWS) scores for each of the seven outcomes (dots). The graph is consistent with most and least counts being inversely related (blue linear regression line). (B) Histograms of variances (VAR) estimated from individual BWS scores. Higher values on the variance scale mean more choice consistency, with lower values meaning less consistency. (C) Histograms of individual BWS scores. These suggest heterogeneous responses, confirming the need to perform cluster analysis.
Figure 2C shows the distribution of BWS scores from participants for each of the seven outcomes. Scores range from a maximum of +3 to a minimum of −3. Positive scores indicate that the outcome is more important, whereas negative scores indicate that the outcome is considered to be less important. A score of +3 indicates that the participant always selected the outcome as being most important, whereas a BWS Score of −3 indicates that the participant always selected the outcome as being least important. Several distributions are non-normal, which suggests that the underlying responses are heterogeneous and that cluster analysis is warranted.
Cluster analysis
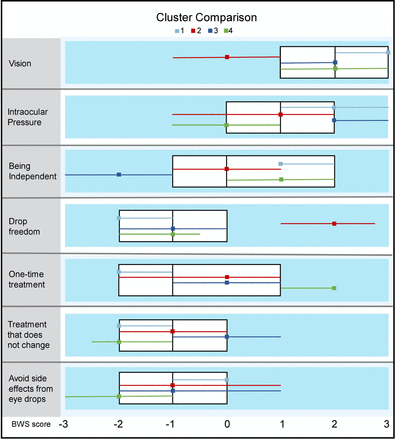
Figure 3 shows that participants form four large clusters with different priorities for health outcomes. In verification of satisfactory cluster formation, the deviance statistic shows that the model is a good fit to the data (p=1.000). That is, individual participants’ BWS scores accurately predict cluster membership.
Cluster analysis of health outcome priorities. Four clusters with different priorities for health outcomes are formed by participants according to their Best–Worst Scaling (BWS) scores. The highest ranked health priority for each cluster is as follows: vision (cluster 1, light blue), drop freedom (cluster 2, red), intraocular pressure and vision (cluster 3, dark blue), one-time treatment and vision (cluster 4, green). BWS scores for each outcome are shown segregated by cluster. More positive scores indicate more important outcomes, whereas more negative scores indicate less important outcomes. For reference, scores for the entire cohort are presented in the white boxplot. Medians are represented by dots (for clusters) and by vertical lines (for entire cohort). IQR is shown by whiskers (for clusters) and box (for entire cohort).
Cluster 1 (vision): This cluster of participants (n=181; 35.4%) assigned the highest priority to the outcome of vision (median BWS Score+3).
Cluster 2 (drop freedom): Participants in this cluster (n=98; 19.2%) rated the treatment-related outcome of drop freedom as most important (median BWS Score+2).
Cluster 3 (intraocular pressure and vision): The third cluster of participants (n=129; 25.2%) assigned highest priority jointly to intraocular pressure and vision (median BWS Score+2).
Cluster 4 (one-time treatment and vision): The final cluster (n=103; 20.2%) prioritised one-time treatment and vision equally (median BWS Score+2).
Multivariate regression model
To determine which variables were associated with each cluster, we used a multivariate logistic regression model that included all sociodemographic and clinical covariates stated in table 1.
Independent predictors of each cluster and their corresponding ORs and 95% CIs are shown in table 2. Cluster 1 (vision) was chosen as the reference cluster. The proportion of missing values was 0.9% and occurred only for data on income and in the records used to obtain disease and treatment data. There were no missing data on self-reported ethnicity or other sociodemographic variables. We conducted a sensitivity analysis to determine whether missing data impacted our analysis. Complete case analysis did not alter our conclusions compared with use of the multiply imputed dataset.
Association of clusters with significant predictors
The most striking finding is that ethnicity was a strong predictor of membership across clusters and thus of health outcome priorities. Ethnicity was the sole significant covariate for cluster 4 (one-time treatment and vision) and the major covariate for cluster 2 (drop freedom).
Cluster 2 (drop freedom): The odds of patients with black/black British ethnicity and other ethnic groups belonging to cluster 2 were 7.31 (95% CI, 3.43 to 15.57) and 4.50 (95% CI, 1.03 to 19.63) times higher, respectively, than white patients. They were much more likely than their white counterparts to choose drop freedom ahead of vision. The duration for which patients had been living with glaucoma had a modest influence on membership of this cluster, with each additional year decreasing the odds by a factor of 0.94 (95% CI, 0.90 to 0.99). This suggests that patients may become slightly more accepting of eye drops with time.
Cluster 3 (intraocular pressure and vision): ORs associating ethnicity with membership of this cluster were 5.95 (95% CI, 2.91 to 12.16) for black/black British, 3.17 (95% CI, 1.12 to 8.96) for Asian/Asian British and 5.37 (95% CI, 1.47 to 19.60) for Other ethnic groups. These patients were much more likely than their white counterparts to assign equal priority to intraocular pressure and vision. For patients with an average annual income of £52 000–£100 000, the OR was 0.07 (95% CI, 0.02 to 0.28), which means that those with this income had 93% lower odds of jointly prioritising intraocular pressure and vision than those with the lowest incomes (<£18 000). Apart from these sociodemographic factors, patients’ treatment history significantly affected the discrimination between cluster 3 and cluster 1. Patients who had received laser treatment had 3.94 (95% CI, 1.17 to 13.29) times higher odds of regarding intraocular pressure to be as important as vision compared with those who had needed eye drops only.
Cluster 4 (one-time treatment and vision): Ethnicity was the only covariate that was significantly associated with this cluster. The OR for prioritising one-time treatment as highly as vision was 2.99 (95% CI, 1.44 to 6.18) for black/black British patients.
Discussion
In this DCE, we found that patients with glaucoma have different priorities for the outcomes of their care. We identified major racial and ethnic disparities in personal priorities, showing for the first time that minority ethnic groups may have differing expectations of the outcomes of care compared with their white counterparts. These differences need to be considered if racial disparities in health outcomes are to be understood and hence equitably addressed.
Collecting data on ethnic groups is complex because of the subjective, multifaceted and changing nature of ethnic identification. It has been pointed out that there is no consensus on what constitutes an ethnic group and membership is something that is self-defined and subjectively meaningful to the person concerned.40 We used contemporaneously self-reported information on ethnicity, in line with recent recommendations.41
Information about ethnic inequalities in health is limited by paucity of data from under-represented populations.42 To ensure that our findings are not unfairly biased against ethnic minority groups, we recruited from the most ethnically diverse region in the UK so that nearly 50% of the participants were from ethnic minority groups.25 We note that large population-based samples such as UK Biobank under-represent individuals with socioeconomic deprivation and from particular ethnic backgrounds, demonstrating that studies of large scale do not necessarily avoid data disparities in which there are systematic differences in the quantity and quality of health data representing different ethnic groups.43
Ethnic disparities in health outcomes may reflect inequalities between ethnic groups in terms of socioeconomic position.7 We have adjusted for the confounding effect of socioeconomic status by including individual-level data on education, income and occupation in our logistic model. We have also corrected for age, gender, disease status and treatment history.
We used real-world data from the patient population. In contrast to prospective trials or case series, we assessed covariates captured from routinely collected medical record data across the whole range of disease severity and treatment history. This maximises the generalisability of our findings to patients routinely seen in glaucoma clinics.
We minimised selection bias by successfully recruiting over 95% of those who were eligible and invited to participate. Our DCE and ethnicity data were complete. Overall, only 0.9% of data were missing, and complete case analysis did not alter our conclusions compared with use of the multiply imputed dataset.
There are limitations in the present analyses. First, preference elicitation using BWS was completed by patients based on their judgement and understanding of hypothetical descriptions. This may involve a cognitive burden for respondents. However, the burden in BWS is lower than traditional ranking DCEs because it is relatively easy to identify the best and worst items of a list.44 Second, patients were asked to make choices between health priorities, all of which had been identified as important in a previous study.34 It may have been difficult for some patients to choose between these priorities because of ambivalence. However, choice consistency as measured by variance was excellent (figure 2B), suggesting that the majority of patients were clear about what really mattered most to them. A third limitation is that we used some retrospectively collected data from medical records and we did not double-check with participants whether the data were correct.
Our findings are consistent with those from previous studies in which intraocular pressure was identified as being important in glaucoma management.45 46 Whereas it was previously reported that intraocular pressure was the top priority for all patients,47 we found that other outcomes were prioritised by different groups of patients. There may be several explanations for the apparent discrepancy. First, Le et al enrolled predominately white patients who may have prevented them from detecting ethnic disparities in preferences. Second, they examined only the aggregated preferences of the whole cohort, and therefore did not check whether there were clusters of individuals with differing priorities. Third, they enrolled patients with early disease who were supposedly suitable for minimally invasive glaucoma surgery, thus limiting elicited preferences to this rather specific group. By contrast, we recruited patients across the broad spectrum of glaucoma severity with varied treatment histories.
The present findings have several important implications. First, patients’ health outcome priorities may not necessarily coincide with their clinicians’ assumptions. This challenges the recent proposal that vision should be the primary outcome in all clinical trials of glaucoma treatment.48 A significant proportion of patients in our study prioritised drop freedom most highly, implying that evaluation of glaucoma treatments should take a bespoke approach, taking each patient’s priorities into account. This supports previous suggestions that patients should define for themselves those aspects of health that impact on QoL, not just in glaucoma but in a variety of clinical settings.49–51 Alternatively, clinicians and researchers would need to use measures of QoL that are validated as being sensitive across the gamut of differing patient priorities. Interestingly, minimally invasive glaucoma surgical procedures have been suggested as a new therapeutic option for glaucoma patients who wish to reduce their medication.52 53 However, evidence that drop freedom is a desired outcome from the patients’ perspective was previously lacking. Our study shows that a significant proportion of patients, but not all, do value drop freedom.
Second, certain treatments may be more suitable for some ethnic groups than others. It was much more likely that black and certain other ethnic groups prized drop freedom as the most important health outcome. The black ethnic group was also more likely to prioritise one-time treatment as highly as vision. Overall, this suggests that these groups would be more likely to benefit from drop freedom produced by one-off treatments such as selective laser trabeculoplasty and minimally invasive glaucoma surgery.52–54 It also helps to explain previous reports that patients from black ethnic groups were less likely to use their glaucoma eye drop medications regularly.55 Thus, identifying patient preferences is important when considering treatment options to maximise concordance with treatment and optimise outcomes, especially in patients with aggressive disease. We speculate that similar disparities in outcome preference may explain ethnic differences in treatment compliance in other areas of medicine.56
Third, our findings suggest that ethnic groups tend to define aspects of their QoL differently. QoL is a multidimensional concept that encompasses opportunity, health perceptions, functional status, impairment and life expectancy.57 Differential priorities for health outcomes may thus explain unexpected dissimilarities found in QoL across ethnic groups in patients with cancer.23 Notwithstanding suggestions that existing ways of measuring QoL are insufficiently sensitive,48 58 the aggregation of the QoL outcomes across different ethnic groups may have masked positive effects of treatment in recent trials.54 59 Furthermore, QoL outcomes from studies which predominantly recruit certain ethnic groups may not be generalizable to other ethnic groups.
It is unknown whether ethnic disparities in priorities for health outcomes exist in other specialisms of healthcare. Regarding the ethnic contrasts demonstrated here, it will be important to determine whether they differ in other geographic regions such that clinicians will need to be aware of the peculiarities of the populations they serve. Longitudinal studies will be required to assess whether individual preferences are stable with time. The reasons underlying the ethnic disparities reported here need further investigation. We cannot exclude the possibility that these disparities may themselves originate in psychological, behavioural and physiological responses of individuals to racism and discrimination.21
Data availability statement
Data are available on reasonable request. The data that support the findings of this study are not openly available to avoid compromising individual privacy. However, anonymised data are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. Approval for the study was granted by the North West—Haydock Research Ethics Committee (REC reference 20/N.W./0347). Participants gave informed consent to participate in the study before taking part.
References
Footnotes
Presented at This work was presented at the Royal College of Ophthalmologists Annual Congress 2023.
Contributors All authors have made substantive intellectual contributions. AS performed the study design, data collection, data analysis and manuscript preparation. KH performed the study design, data interpretation and manuscript preparation. EK performed data interpretation and manuscript critique. GG performed study design and manuscript critique. KH and GG had supervisory roles and oversaw administrative and financial aspects. KH is the study guarantor.
Funding This study was supported by a grant to AS from Ministry of Finance of Republic of Indonesia through Indonesia Endowment Fund for Education (Grant number 201901220413853). The funder had no role in study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit for publication. This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests All authors declare: support from Indonesia Endowment fund for Education (LPDP) and NIHR Moorfields Biomedical Research Centre for the submitted works. GG declares grants or contracts from any entity (Thea, Santen—unrestricted research grants); consulting fees (Alcon, Allergan, Belkin, Elios, Equinox, Genentech/Roche, Glaukos, Ivantis, McKinsey, Rayner, Reichert, Ripple Therapeutics, Santen, Sight Sciences, Thea, Vialase, Visufarma, Zeiss); payment or honoraria for lectures, presentations, speakers’ bureaux, manuscript writing or educational events (Alcon, Allergan, Belkin, Glaukos, Ivantis, Lumibird, McKinsey, Reichert, Sight Sciences, Thea); support for attending meetings and/or travel (Ivantis, Thea); leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid (Clinical Advisory to Patient Advocacy Group Glaucoma UK; President of UK & Ireland Glaucoma Society). EK declares lecture Honoria (AbbVie) and support for attending meetings (University of West Attica). Other authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. The lead author (KH) affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.