Article Text
Abstract
Objectives To compare catheter-related outcomes of individuals who received a tunnelled femorally inserted central catheter (tFICC) with those who received a peripherally inserted central catheter (PICC) in the upper extremities.
Design A propensity-score matched cohort study.
Setting A 980-bed tertiary referral hospital in South West Sydney, Australia.
Participants In-patients referred to the hospital central venous access service for the insertion of a central venous access device.
Primary and secondary outcome measures The primary outcome of interest was the incidence of all-cause catheter failure. Secondary outcomes included the rates of catheters removed because of suspected or confirmed catheter-associated infection, catheter dwell and confirmed upper or lower extremity deep vein thrombosis (DVT).
Results The overall rate of all-cause catheter failure in the matched tFICC and PICC cohort was 2.4/1000 catheter days (95% CI 1.1 to 4.4) and 3.0/1000 catheter days (95% CI 2.3 to 3.9), respectively, and when compared, no difference was observed (difference −0.63/1000 catheter days, 95% CI −2.32 to 1.06). We found no differences in catheter dwell (mean difference of 14.2 days, 95% CI −6.6 to 35.0, p=0.910); or in the cumulative probability of failure between the two groups within the first month of dwell (p=0.358). No significant differences were observed in the rate of catheters requiring removal for confirmed central line-associated bloodstream infection (difference 0.13/1000 catheter day, 95% CI −0.36 to 0.63, p=0.896). Similarly, no significant differences were found between the groups for confirmed catheter-related DVT (difference −0.11 per 1000 catheter days, 95% CI −0.26 to 0.04, p=1.00).
Conclusion There were no differences in catheter-related outcomes between the matched cohort of tFICC and PICC patients, suggesting that tFICCs are a possible alternative for vascular access when the veins of the upper extremities or thoracic region are not viable for catheterisation.
- intensive & critical care
- internal medicine
- vascular medicine
- infection control
Data availability statement
Data are available upon reasonable request. Data availability and access subject to local health district review of request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Propensity score matching was used to minimise bias in baseline characteristics and improve comparability between the peripherally inserted central catheter (PICC) and tunnelled femorally inserted central catheters (tFICC) cohorts.
A diverse range of patients requiring either PICC or tFICC insertion were included in this study, increasing the validity of study results.
One limitation of this study is its retrospective nature and being conducted at a single centre, this may limit the generalisability of study results.
Potential for residual confounding due to unmeasured variables influencing propensity score analysis.
Background
The majority of central venous access best practice guidelines recommend against using the common femoral vein (CFV) except in emergencies or for short-term use.1–5 Compared with centrally inserted central catheters (CICCs) and peripherally inserted central catheters (PICCs), CFV catheters are more likely to colonise because of the humidity and increased density of skin flora in this region. Additionally, maintaining sterile dressing adherence can pose a challenge that can increase the risk of developing a central line-associated bloodstream infection (CLABSI).4–9
Thrombosis has also been a historical concern with CFV catheters. A higher incidence of semiocclusive and complete occlusive thrombosis in the critically ill from CFV catheters has been reported compared with CICCs.6 10–13 The incidence of iliofemoral thrombosis associated with CFV catheterisation can be as high as 25%. The risk of developing this condition is greater when larger bore catheters are used.14 15
However, there are situations when a patient’s anatomical variance or clinical condition necessitates vascular access via the CFV.4 16 17 Traditional CFV catheters are inserted in the groin close to the inguinal ligament, where the high-density microbial load increases the risk of catheter colonisation.16 To reduce the risk of infection and premature failure (through better dressing adherence and catheter stabilisation), a practice adopted by vascular access teams and specialties like neonatology is to move the catheter exit site away from the groin.17–20
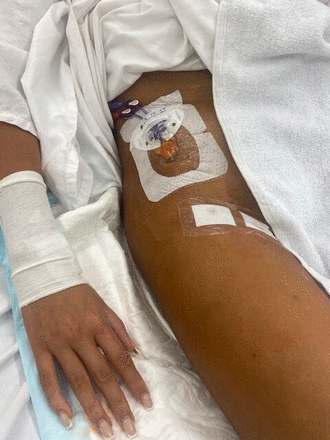
Unlike standard 20–25 cm CFV catheters (that usually terminate in the iliac vein), tunnelled femorally inserted central catheters (tFICCs) are 50–60 cm (typically standard PICCs), that terminate in the inferior vena cava and do not require the skin exit site to be at the venepuncture site. A tFICC exit site can be planned depending on patient requirement (mobilisation needs, continence, delirium with risk of accidental dislodgement),21 22 it can be inferior to the venepuncture site with the catheter exiting at the mid-thigh (figure 1) in both the supine or prone position or superior to the venepuncture site, exiting on the abdomen (figure 2).23
tFICC exiting at the mid-thigh. tFICC, tunnelled femorally inserted central catheter.
FICC tunnelled and exiting on the abdomen. FICC, femorally inserted central catheter.
Positioning the tFICC exit site away from the groin can be achieved by two methods. The first method involves puncturing the skin distal to the inguinal crease with a 10 cm needle to access the CFV (pseudo-tunnelling technique) while the second method involves skin puncture closer to the groin then simple subcutaneous tunnelling to the desired exit site (traditional tunnelling technique). The choice of technique is dependent on patient body habitus, depth and characteristics of the target vessel and desired distance of the catheter exit site from the groin. Inserting PICCs with French (F) sizes that range from 4 F (single lumen) to 6 F (triple lumen) for adults in the CFV, better accommodates catheter size to vein diameter ratio, a known modifiable risk factor for endothelial irritation, reduced blood flow and thrombosis.3 24 25
Because of the potential advantages of tFICCs, they have been considered a possible substitute to standard CFV catheters for patients requiring intermediate, and even long-term venous access when catheterisation of the upper extremities and central veins are not an option.16 19 26–28 We hypothesised that catheter-related outcomes of individuals receiving a tFICC would be no worse than those individuals receiving a traditional PICC via the upper extremities. Our study, therefore, aimed to compare catheter-related outcomes of tFICCs and PICCs by propensity score matched analysis.
Methods
Study design and population
The source population for the study was a retrospective cohort of patients requiring a central venous access device (CVAD), at our 980-bed tertiary referral hospital in South West Sydney, Australia, between January 2014 and December 2020. The hospital provides clinical services to a large geographic area and caters to a diverse population. The Strengthening the Reporting of Observational Studies in Epidemiology statement was used to guide the study’s design, data collection, analysis, results and conclusions.29
For our analysis, we included adult patients (18 years or older) referred to the hospital central venous access service (CVAS). This service comprises specialist vascular access nurses who are responsible for inserting acute and chronic CVADs for all patient groups in the hospital. Patients were excluded from enrolment if they were under the age of 18 or received a CVAD other than a PICC in the upper extremity or tFICC. Participants for this study were identified through the CVAS operational database. There were no planned data imputation for any missing data.
During the study period, a tFICC was inserted if the vessels in the upper extremities and central veins were found to be unsuitable for catheterisation due to venous depletion, significant thrombosis or anatomical variance (such as mediastinal lymph compression of the superior vena cava). Prior to tFICC insertion, the safety and utility of inserting the catheter were determined after routine ultrasound assessment of the veins in the femoral region and distally towards the mid-thigh area. This standard assessment process guided the decision for the most appropriate venepuncture site and type of tunnelling required (pseudo vs traditional tunnelling).21 22
For all PICC and tFICC insertions, we inserted standard 55 cm polyurethane pressure injectable catheters. Clinical management protocols of all CVADs were based on hospital policies and guidelines and were not distinguished between anatomical insertion sites. All patients were followed from device insertion until catheter removal as part of the CVAS routine surveillance for hospital CVAD complications.
The primary outcome of interest was the rate of all-cause catheter failure, a composite of failure resulting in catheter removal. This included catheter occlusion, partial or complete dislodgment, catheter fracture, catheter-related thrombosis and CLABSI. Secondary outcomes were rates of catheters removed because of suspected catheter-associated infection (composite of suspected blood stream, catheter exit site and or tunnel infection), confirmed CLABSI as defined by the National Healthcare and Safety Network (a primary bloodstream infection in a patient who had a central line within the 48-hour period before the development of the infection),30 device dwell time (time from CVAD insertion to removal in days) and confirmed catheter associated upper or lower extremity deep vein thrombosis (DVT, defined as a formally reported thrombosis by a suitably qualified sonographer or medical practitioner).
Statistical analysis
The characteristics of study participants for continuous data are presented using a 6-point descriptive summary (n, mean, SD, median and IQR (25th and 75th percentiles)) and categorical data are described using frequencies and proportions. To minimise differences in characteristics between groups, that may potentially confound the relationship between the insertion of a tFICC or a PICC and catheter outcomes, patients were matched using a propensity score for receiving a tFICC. Importantly, predictors (propensity) for a tFICC were identified using a stepwise (both backward and forward) approach, with a liberal p value of 0.10 for inclusion, using a binary logistic regression model. Final predictors were (1) age; (2) sex; (3) indication for a CVAD; (4) history of diabetes; (5) having an oncology diagnosis; (6) renal disease; (7) one or more comorbid conditions and (8) the number of previous CVADs. To ensure adequate power of our final sample size, using these eight predictors, we attempted to match up to four PICC patients for each tFICC, using the nearest neighbour approach within a calliper distance (ie, SD of logit of the propensity score) of 0.2. All matching was undertaken using the R matchIt package.31 32 As a result, based on an overall proposed baseline failure rate of 15%, and a non-inferiority boundary of 10% difference between the two groups, our final sample size was estimated to have a power of at least 0.80, with type I error set at 5%.
The primary and secondary outcomes of interest are presented with the estimated differences between the tFICC and PICC groups, with associated 95% CIs. P values for the comparison between tFICC and PICC groups were obtained using linear or generalised linear mixed effect models, clustered at the 1:4 matching and individual patient level to account for repeated catheters. Similarly, the average catheter dwell times and 95% CIs were estimated using a linear mixed effect model. The cumulative probabilities of all-cause failure for the FICC and PICC groups were estimated and presented using the approach suggested by Kaplan-Meier and the differences in failure curves were compared using Cox’s Proportional Hazards model, adjusting for catheter diameter and number of lumens.33 All data management and analyses were conducted using the R language for Statistical Computing.34 tFICCs were not distinguished by tunnelling method for analysis.
Patient and public involvement
None.
Results
From January 2014 to January 2020, the hospital’s CVAS inserted 4268 PICCs. During this study period, they also inserted 98 tFICCs for 77 patients, some of whom required more than one tFICC for the same or separate hospital admission. All patients were included in the analysis. Before performing propensity score matching, we found significant differences between the two groups (table 1). Specifically, the tFICC group had almost two times the proportion of patients with primary renal disease (n=17, 17%) compared with the PICC group (n=364, 9%, p=0.002). The primary reason for requiring a tFICC was difficult venous access, which accounted for 44% of cases (n=43), while only 4% of patients in the PICC group (n=164, p=<0.001) needed a catheter for this reason. After propensity score matching, no differences in baseline characteristics were observed between the 98 tFICCs and the 385 closest-matched patients in the PICC (control) group (table 1).
Baseline characteristics before and after propensity score matching of PICC and tFICC patients
After analysing catheter outcomes in the matched cohort, we observed no statistical difference in our primary outcome of interest, all-cause catheter failure (difference −0.63 per 1000 catheter days, 95% CI −2.32 to 1.06) (table 2). We found the PICC group to have longer average dwell (58 mean days, 95% CI 43.8 to 72.4) than the tFICC group (43.8 mean days, 95% CI 28.5 to 59.0). However, we did not find any significant difference in catheter dwell time between the two groups (mean difference of 14.2 days, 95% CI −6.6 to 35.0, p=0.910, table 2). Furthermore, we observed non-differences in the cumulative probability of failure between the tFICC and PICC groups, during the first month of dwell (figure 3).
Cumulative probability of all-cause catheter failure between FICC and PICC groups in the first month of dwell using the Cox’s PH model adjusted for catheter diameter and number of lumens. FICC, femorally inserted central catheter; PICC, peripherally inserted central catheter.
Outcomes of tFICCs and propensity score matched PICCs
Both groups showed a low rate of catheters requiring removal due to suspected catheter-associated infection or confirmed CLABSI. When the rates of removal for confirmed CLABSI were calculated, there was no statistically significant difference found between the two groups (difference of 0.13 per 1000 catheter days, 95% CI −0.36 to 0.63, p=0.896). Similarly, there was no significant difference observed between the two groups with regards to catheters requiring removal for symptomatic thrombosis (difference of −0.11 per 1000 catheter days, 95% CI −0.26 to 0.04, p=1.00). (table 2).
Discussion
Patients with complex medical conditions, such as multimorbidity, musculoskeletal contractures, haematology/oncology disease or the critically ill, are more likely to have challenging venous access where conventional methods of vascular access may be inappropriate or inadequate.22 26 35 Inserting tFICCs in such cases can be considered a reasonable alternative. When we compared catheter-related outcomes with our matched cohort, we found no differences in failure rates, infectious outcomes or thrombotic events between patients who received a PICC in the upper extremities or a tFICC in the lower extremity.
The rates of all-cause failure for tFICCs (2.4/1000-line days) and for PICCs (3.0/1000-line days) in this study were comparatively low when previously published literature was considered. A prospective cohort study that evaluated rates of PICC complications reported an overall failure rate of 14.38/1000-line days and a systematic review examining failure rates of CVADS (by device type) in intensive care units, reported a PICC failure rate of 3.98/1000-line days (six studies; 13 078 catheter days).36 37 A study comparing femoral catheter exit sites found catheters exiting at the groin had higher overall complication rates than those exiting mid-thigh (45% vs 12% p<0.001).38
Catheters that exit near the groin are at higher risk of CLABSI.6 9 39 This can result in additional organisational treatment costs of up to US$ 50 000 per episode as well as a near threefold increase in mortality risk and longer hospital length of stay.40 Ensuring optimal sterile dressing adherence over catheter in the groin region is, therefore, critical, although this can be challenging and can lead to subsequent unplanned dressing changes. Studies have shown that more than two interrupted dressing changes increase the risk of CLABSI threefold with the risk for infection greater with femoral sites compared with others.41 42
The relocation of the catheter exit site, away from the groin in our study, likely improved catheter dressing adherence and contributed to the low tFICC CLABSI rate (0.24/1000-line days) that was comparable to our PICC rates (0.11/1000-line days). When considering the published literature, CLABSI rates for PICCs have been reported at up to 2.5/1000-line days and 3.6/1000-line days for traditional CFV catheters.8 37 Our tFICC CLABSI rate was also lower compared with other observational studies that reported infectious outcomes with tFICCs. A study of 600 patients with multidrug-resistant bacterial strains, which evaluated infections of tFICCs exiting mid-thigh, reported a CLABSI rate of 0.46 per 1000-line days. Similarly, a feasibility study that assessed the safety and utility of tFICCs reported a CLABSI rate of 1.28 per 1000-line days.19 43
We reported near negligible symptomatic DVT rates in the matched group. While asymptomatic thrombosis cannot be discounted, no catheters required removing for symptomatic DVT in the tFICC group and only two in the PICC group (0.5%, 0.11/1000-line days). Symptomatic DVT rates in adult patients have been reported to vary from less than 1% to 28%.44–47 Our systematic approach to vascular assessment, choice of catheter size based on vessel diameter and insertion technique (ultrasound guidance and optimal tip location using intracavitary electrocardiography) possibly contributed to this low rate.21 48 49 A study by Greene and colleagues reported thromboembolic events were lower with PICCs inserted in the lower extremities (HR, 1.48; 95% CI 1.02 to 2.15) compared with those inserted in the upper extremities (HR, 10.49; 95% CI 7.79 to 14.11).50 In a retrospective cohort study of 874 patients with superior vena cava syndrome who received a tFICC for chemotherapy, the overall incidence of DVT was 16.47% (0.91/1000-line days), with symptomatic DVT reported at 2.01% (0.1/1000-line days).13
During the study period, we found that the total number of tFICCs inserted was much lower compared with total PICCs (98 FICCs vs 4268 PICCs). The low rate of tFICC insertion reflected patients who presented to the CVAS for whom traditional CVAD insertion was not possible. This highlights that tFICC insertion is not the primary choice in most clinical cases but rather an alternative option when necessary.
However, in the paediatric and infant populations, FICC insertion is an established practice and has been used successfully as a first-line approach and when upper extremity vessels and central veins are unsuitable for cannulation.51 52 In the neonatal population, FICCs have been reported to be a safer alternative to traditional CVAD placement by preserving upper extremity vessels and by reducing iatrogenic risks associated with CICC insertion that includes prolonged sedation, radiation exposure associated with fluoroscopic guidance and supradiaphragmatic central venous insertion.53 54
Examining the utility of tFICCs and their role in providing secure vascular access for chronic and complex patients who present to hospitals with challenging venous access is vitally important.55 56 In 2020, more than half of all hospitalisations in Australia (52%) were due to chronic and complex diseases, resulting in 5.8 million admissions.57 Globally, individuals with chronic and complex conditions are 14 times more likely to require hospitalisation and greater healthcare utilisation. Therefore, it is crucial to explore appropriate alternative solutions to traditional vascular access techniques for people with chronic and complex disease.58
While FICC insertion may be a suitable vascular access solution for some patients, it may not necessarily be the right choice for all. People with chronic kidney disease, particularly those preserving upper limb and central veins for future fistula formation or long-term dialysis catheter insertion, may find tFICC insertion beneficial. However, those anticipating or having undergone a kidney transplant, tFICC insertion demands cautious evaluation due to potential complications arising from the surgical anastomosis of the femoral vessels to the transplanted kidney. Therefore, thorough clinical assessment is imperative before considering tFICC insertion to identify and manage any contraindications.
Strength and limitations
We also acknowledge there are limitations to this study. First, it was a retrospective cohort study that was conducted at a single centre. The estimation of the effects of tFICCs, in comparison to PICCs inserted in the upper extremities, is subjected to confounding. However, we have attempted to overcome this issue by using a propensity score method that allowed us to match the baseline characteristics in the tFICC and PICC groups. This method helped us to compare patients with the same or similar value of propensity score. This type of balancing is similar to that provided by randomised control trials, with the key difference being that the propensity score balances the two groups, whereas in randomised controlled trials, balancing occurs through the process of randomisation.59
Reported baseline characteristics were also limited to data availability from the hospital’s clinical and administrative databases (and the CVAS operational database). The CVAS at the study hospital almost exclusively insert all PICCs and tFICCs. As such, it is likely that specific clinical conditions such as coagulopathy, sepsis and critical illness were represented in both groups; however, this cannot be guaranteed, and such unaccounted variables could have led to residual confounding that influenced the propensity score analysis. Thus, the reported outcomes of our study may not be generalisable across other populations.
Conclusion
In hospitalised patients who require intermediate to long-term venous access for whom traditional methods of vascular access are not adequate or contraindicated, the insertion of a tFICC with the exit site away from the inguinal region can be a safe and reliable vascular access alternative. However, larger prospective studies comparing tFICCs to traditional sites in specific population groups such as those with renal disease are needed to further establish the effectiveness of tFICCs.
Data availability statement
Data are available upon reasonable request. Data availability and access subject to local health district review of request.
Ethics statements
Patient consent for publication
Ethics approval
Approval to perform this study was provided by the Liverpool Hospital quality and safety committee and included wavering consent (reference number: LIV81/2019/34).
References
Footnotes
Contributors CM, NM, SF, MO and EA contributed to study conception and design. RR, SV TT and NM contributed to data collection. CM, SF, LH and EA contributed to data analysis and interpretation of results. CM, MO, LH, SF and EA contributed to drafting manuscript. EA is the guarantor for this study and accepts full responsibility for the work and/or the conduct of the study, had access to the data and controlled the decision to publish. All authors contributed to editing the manuscript and approved the final version for publishing as per the International Committee of Medical Journal Editors (ICMJE) convention.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer-reviewed.