Article Text
Abstract
Objective To characterise sex and gender-based analysis (SGBA) and diversity metric reporting, representation of female/women participants in acute care trials and temporal changes in reporting before and after publication of the 2016 Sex and Gender Equity in Research guideline.
Design Systematic review.
Data sources We searched MEDLINE for trials published in five leading medical journals in 2014, 2018 and 2020.
Study selection Trials that enrolled acutely ill adults, compared two or more interventions and reported at least one clinical outcome.
Data abstraction and synthesis 4 reviewers screened citations and 22 reviewers abstracted data, in duplicate. We compared reporting differences between intensive care unit (ICU) and cardiology trials.
Results We included 88 trials (75 (85.2%) ICU and 13 (14.8%) cardiology) (n=111 428; 38 140 (34.2%) females/women). Of 23 (26.1%) trials that reported an SGBA, most used a forest plot (22 (95.7%)), were prespecified (21 (91.3%)) and reported a sex-by-intervention interaction with a significance test (19 (82.6%)). Discordant sex and gender terminology were found between headings and subheadings within baseline characteristics tables (17/32 (53.1%)) and between baseline characteristics tables and SGBA (4/23 (17.4%)). Only 25 acute care trials (28.4%) reported race or ethnicity. Participants were predominantly white (78.8%) and male/men (65.8%). No trial reported gendered-social factors. SGBA reporting and female/women representation did not improve temporally. Compared with ICU trials, cardiology trials reported significantly more SGBA (15/75 (20%) vs 8/13 (61.5%) p=0.005).
Conclusions Acute care trials in leading medical journals infrequently included SGBA, female/women and non-white trial participants, reported race or ethnicity and never reported gender-related factors. Substantial opportunity exists to improve SGBA and diversity metric reporting and recruitment of female/women participants in acute care trials.
PROSPERO registration number CRD42022282565.
- INTENSIVE & CRITICAL CARE
- Health Equity
Data availability statement
Data are available on reasonable request. Complete statistical analysis plan and raw data are available on author request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Broad search strategy, duplicate citation screening and data abstraction, and adjudication of sex and gender-based analysis (SGBA) reporting with a statistician.
First systematic review examining SGBA and diversity metric reporting in acute care randomised controlled trials published in high-impact general medical journals.
Inclusion of a diverse sample of acute care trials before and after Sex and Gender Equity in Research guideline publication.
Search restricted to selected journals and publication years, with assumption that if SGBA and diversity metric reporting were suboptimal in these high-impact journals, it would be of similar or lower quality in other journals.
We did not consider sex-specific disease prevalence or power issues related to SGBA.
Introduction
Biological sex and sociocultural gender are key determinants of health influencing all aspects of disease development and progression.1 Sex-related differences in physiology, pharmacology, disease prevalence and underlying pathophysiology are well described.2–9 Gender, as complex social construct, and gendered-social factors, including education level and employment status, have been increasingly recognised as important factors in health.6 More recently, there has been increasing recognition of the intersection between sex, gender and other factors such as race or ethnicity and their impact on health.6 10 11 Notwithstanding, females and women are under-represented as participants in clinical trials4 5 7 8 12 and statistical analyses infrequently address the impact of these variables on dosing, treatment effect and adverse events.3 4 7
As early as 2007, researchers highlighted the need for reporting the primary outcome of trials disaggregated by sex or gender with a test of interaction to provide more equitable and inclusive evidence.9 13 These have been defined as sexand gender-based analyses (SGBA). In 2013, the International Committee of Medical Journal Editors recommended routine reporting of data by sex.14 In 2016, the Sex and Gender Equity in Research (SAGER) guideline was published to standardise and promote sex and gender reporting.4 In acute care medicine, the implementation of sex-sensitive and gender-sensitive research remains an unmet need.15 A 2011 review of 2336 diverse Emergency Medicine studies found that although 29% of authors considered sex or gender in their study design, only 2% reported their primary outcome by sex or gender.16 A 2018 update of this study found that although the incorporation of sex and gender in the study design increased over time, the proportion taking sex or gender into consideration when reporting their primary outcome remained unchanged.17 The effect of the SAGER guideline on reporting of acute care trials in leading medical journals is unknown.
We performed a systematic review to characterise reporting of SGBA, diversity metrics (ethnicity, race, gender-related factors) and the proportion of female/women participants included in acute care randomised controlled trials published in high-impact medical journals. We further assessed whether SGBA reporting and inclusion of female/women participants improved over time.
Materials and methods
Objectives
Our primary objectives were to characterise reporting of SGBA and representation of females/women in acute care trials. In secondary objectives, we aimed to describe diversity metric reporting (ie, ethnicity, race, income, education, marital status, employment status) and assess whether SGBA reporting and inclusion of females/women improved after publication of the SAGER guideline. This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations18 and was registered on PROSPERO (CRD42022282565).
Data sources and searches
We systematically searched MEDLINE for acute care trials published in five journals including the Journal of the American Medical Association (JAMA), New England Journal of Medicine (NEJM), British Medical Journal (BMJ), The Lancet and Annals of Internal Medicine in 2014, 2018 and 2020. These journals were selected as seminal acute care trials are frequently published in these journals, and they rank among the top five general medical journals when sorted by h-index. Additionally, we theorised that if reporting of SGBA and diversity metrics was suboptimal among these selected leading general medical journals with high reporting standards, reporting would likely be suboptimal in other general medical journals and subspecialty journals. We selected these years to identify trials published before and after the 2016 SAGER guideline.4 The search used keywords “Randomized Controlled Trial” or “Controlled Clinical Trial” or “Pragmatic Clinical Trial” or “Equivalence Trial” or “Clinical Trial, Phase III” regardless of their focus or language of publication (online supplemental material: search strategy).
Supplemental material
Trial selection
We included parallel group trials that enrolled acutely ill (at least 50% acutely ill) adults (age greater than 18 years), compared two or more interventions or strategies and reported at least one clinical outcome (ie, mortality, length of stay. We defined acutely ill as necessitating admission to an intensive care unit (ICU) or receiving treatments typically initiated in the ICU with expected impact on short-term and long-term outcomes. Patients with an unstable cardiac diagnosis (eg, heart failure exacerbation, acute coronary syndrome) requiring hospitalisation were also considered acutely ill. Trials that assessed cardiology interventions or patients that would typically be admitted to a coronary care unit or cardiology ward were considered as ‘cardiology trials’. All other trials were considered to be ‘ICU trials’. We excluded case reports, case series, observational studies, cross-over, n of 1, cluster and quasi-randomised trials. Further, we excluded trials if the intervention was administered exclusively in the prehospital setting, emergency department or operating room and patients were not subsequently admitted to an ICU or monitored setting. Trials that enrolled predominantly outpatients, non-adults, evaluated elective procedures (eg, elective cardiac surgery or percutaneous coronary intervention) or included more than 50% inpatients who were not acutely ill at the time of treatment administration were also excluded.
Four reviewers (DG, AA, KH and JOF), working in pairs, screened citations initially by title and abstract and subsequently, by full text, independently and in duplicate. Disagreements were adjudicated by four investigators (KEAB, JOF, KH and DG). All citations were screened using Covidence software.19
Data abstraction and quality assessment
22 reviewers, mostly methodologists, (DG, AA, KH, JOF, BP, RS, VF, GR, MK, SV, DC, CG-B, FD’A, DW, VIL, CL, JR, VT, VP, EB-C, MAM and KEAB), working in pairs, abstracted data independently and in duplicate using a standardised data abstraction form. Disagreements were resolved by adjudication by two investigators (DG and KEAB).
We abstracted data related to trial design (objective, primary outcome, location), funding, participant diversity (sex, gender, race, ethnicity, income etc), if an SGBA for the primary outcome was performed, details related to the SGBA (specified a priori in the methods section of included trials, depicted using a forest plot, corrected for multiple comparisons and whether a sex-by-intervention interaction was performed with an accompanying frequentist or Bayesian test of significance).9 13 We also noted whether trials featured a sensitivity analysis by sex. We did not consider reporting of sex or gender as a covariate in an adjusted analysis to be a valid SGBA.20 Trials needed to report both a treatment and subgroup variable to be considered a SGBA. We considered analyses that assessed for a sex-by-intervention interaction to represent more robust SGBA.9 13 20 21 We examined online supplemental materials and appendices of all included trials to ensure that SGBAs were not missed. A graduate student in statistics (MR) working with a biostatistician (LT) confirmed SGBA reporting and features of SGBA.
We recorded terms used by trial authors to report sex or gender in headings (ie, sex or gender) and subclassifications (eg, male/female/other, man/woman/other) in baseline characteristics tables and SGBA. We assessed for concordance (sex subclassified as female/male/other or gender subclassified as man/woman/other) between table headings and sex/gender subclassifications within baseline characteristics tables. Any other combination of terminology between headings and subheading was deemed discordant terminology. In trials that reported SGBA, we noted if subclassification terms were inappropriately used interchangeably between baseline characteristics tables and SGBA. We did not assess for concordance of race and ethnicity terminology throughout included trials, however, we did assess if these were appropriately presented as distinct entities within baseline characteristics tables.
We used the Gender Outcomes International Group: to Further Well-being Development framework to characterise diversity metrics and domains encompassed by gender such as gender identity, gender relations, gender roles and institutionalised gender.6 21–23 We collected data regarding participant gendered-social factors including income, education, marital or employment status. We documented how race and ethnicity were reported and the number of trial participants by category. Finally, we noted whether trials discussed the implications of SGBA when conducted or identified the absence of an SGBA as a limitation. We did not assess trial risk of bias as our goal was to focus on SGBA and diversity reporting in selected high-impact medical journals.
Subgroup analyses
A priori, we planned to compare SGBA reporting in ICU versus cardiology trials.
Statistical analysis
We used descriptive statistics including counts and proportions, means and SD to summarise binary and continuous data, respectively. We used the χ2 test with Yates’ correction to compare: (1) SGBA reporting before and after the publication of the 2016 SAGER guideline and (2) SGBA reporting in ICU versus cardiology trials. All analyses were conducted in WinPepi24 and Stata MP (StataCorp, V.17). We created figures using Microsoft Excel and Stata.25 All statistical analyses were performed with a level of significance set at p=0.05.
We tabulated the pooled proportion of females/women: (1) in cardiology and ICU trials, (2) by publication year and (3) before (2014) versus after SAGER (2018 and 2020) guideline publication using the metaprop command in Stata, with random-effects models.26 We assessed whether the proportion of female/women trial participants differed before and after SAGER guideline publication using meta-regression (R2) using the meta command with regress subcommand in Stata in a random-effects model.
Deviations from preregistered protocol
While we largely adhered to our preregistered PROSPERO protocol, methods that were not identified in our initial protocol include the use of the GOING-FWD23 framework to characterise diversity metrics, evaluating the concordance of sex and gender terminology, subgroup analyses comparing female/women inclusion in cardiology versus ICU trials, and statisticians (MR and LT) confirming features of included trial SGBA.
Patient and public involvement
Members of the public and patients were not involved in the design, interpretation or dissemination of this study.
Results
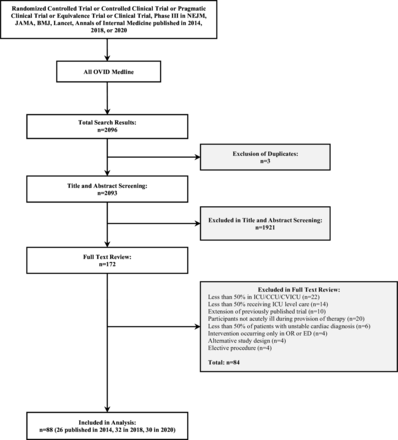
After removal of duplicates, we identified 2093 citations for title and abstract review. We excluded 1921 citations, leaving 172 trials for full-text review. Of these, 88 trials met inclusion criteria including 75 (85.2%) ICU and 13 (14.8%) cardiology trials (figure 1). Four trials required adjudication by KEAB and DG. Most trials were multicentre (83 (94.3%)) and 55 (62.5%) trials included less than 1000 participants. Trials were predominantly from Europe (49 (55.7%)) and North America (24 (27.3%)) and published in NEJM (35 (39.8%)), JAMA (35 (39.8%)) and The Lancet (17 (19.3%)) (table 1). A similar number of trials were included across each of the years of publication. Acute care trials typically evaluated cardiovascular, neurologic or respiratory interventions. Of these, more than half (50 (56.8%)) were pharmacological interventions.
Characteristics of acute care trials
Trial identification. BMJ, British Medical Journal; CCU, coronary care unit; CVICU, cardiovascular intensive care unit; ED, emergency department; ICU, intensive care unit; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; OR, operating room.
Reporting of sex-based and gender-based analyses
23 (26.1%) trials reported an SGBA of which most were prespecified (21/23 (91.3%)) and depicted in a Forest plot (22/23 (95.7%)). Most SGBA (19/23 (82.6%)) reported a sex-by-intervention interaction with an associated Frequentist or Bayesian test for significance. Five trials (5.7%) included a sensitivity analysis based on sex. Only one trial discussed the implications of SGBA on the primary outcome. Of the trials that did not conduct an SGBA, none identified the lack of an SGBA as a limitation.
Seven of 26 trials (27%) published in 2014 conducted SGBAs, while 8/32 (25%) trials in 2018 and 8/30 (27%) trials in 2020 reported SGBAs (figure 2). There was no difference in the proportion of trials that reported SGBAs before and after publication of the SAGER guideline (7/26 (27%) vs 16/62 (25.8%); p=0.88). Significantly fewer SGBAs were reported in ICU vs cardiology trials ((15/75 (20%) vs 8/13 (61.5%) p=0.005).
Reporting of sex and gender-based analyses (SGBA) in acute care trials over time.
Sex or gender of included trial participants
There were 111 428 total trial participants, including 38 140 (34.2%) females/women and 73 288 (65.8%) males/men. Only one trial included an ‘other’ category—characterising a participant as ‘living as female’.27 There were more female/women participants in ICU (30 903 (37.1%), 75 trials; n=83 199) vs cardiology trials (7237 (25.6%); 13 trials; n=28 229), (p<0.001). Similar findings were observed in the pooled prevalence of females/women in ICU versus cardiology trials (p=0.005). There were no differences in the pooled prevalence of female/women participants across publication years (p=0.62) and before versus after SAGER guideline publication (p=0.59) (online supplemental figures 1–3). Meta-regression evaluating female/women representation across years indicated no improvement over time (R2 of 1.01%). (online supplemental figures 4–5).
Supplemental material
Sex and gender reporting
In table 2, we summarise the terminology used to report participant sex and gender in baseline characteristics tables and SGBA. Of the 32 trials that featured a sex or gender heading in their baseline characteristics table, most (31 (96.9%)) reported sex, only 1 (3.1%) trial reported gender. Of these trials, 17 (53.1%) used discordant terminology between the heading and subclassification within baseline characteristics tables. Four (17.4%) trials used sex and gender subclassifications interchangeably between baseline characteristics table and SGBA.
Use of sex and gender terminology in reporting acute care trials
Race and ethnicity reporting
Race and ethnicity were usually reported as distinct entities. Only 25 (28.4%) trials reported race or ethnicity (table 3). Of these, one trial had incomplete data,28 two trials did not report race and ethnicity as distinct entities,27 29 one trial reported trial participants as ‘black’ or ‘not black’30 and another trial did not report mutually exclusive race categories,31 precluding pooling of these trials (table 3). Among the remaining 20 trials that reported race or ethnicity, participants were predominantly (78.8%) white. Of these, six trials categorised participants as ‘white’ or ‘not white’.32–37 We did not find significant differences in race and ethnicity reporting between ICU and cardiology trials (5/13 (38.5%) vs 20/75 (26.7%); p=0.4).
Reporting of race and ethnicity in acute care trials
Reporting of gendered-social factors
No trial reported gendered-social factors.
Discussion
Despite reporting recommendations, SGBAs were infrequently reported among our sample of acute care trials published in high-impact medical journals over a 7-year period. Only one-third of acute care trial participants were females/women. Most trials that included a sex or gender heading in their baseline characteristics table reported participant sex. Discordant sex and gender terminology were noted in over half of the included trials between headings and subheadings within baseline characteristics tables, and in approximately 20% of trials between baseline characteristics tables and SGBA. Nearly 80% of acute care trial participants were white. Less than 30% of acute care trials reported race or ethnicity. No trial reported on income, education, marital status or employment status. Only one trial featured an ‘other’ category in their sex or gender demographic reporting, which included one participant. SGBA reporting and inclusion of female/women participants did not improve over time. Compared with ICU trials, cardiology trials reported significantly more SGBA. It is unclear why reporting of SGBA has not improved over time despite publication of the SAGER guideline. Possible explanations include delays in knowledge dissemination and time required for guideline adoption (as trials may have been designed and conducted several years prior to publication), lack of enforcement by journal editors and peer reviewers, concerns regarding multiplicity and false positives in subgroup testing, and the effect (real or perceived) sex-specific disease prevalence may have on the decision to conduct SBGA. Substantial opportunity exists to improve SGBA and diversity metric reporting and recruitment of female/women participants in acute care trials.
Our study has several strengths including a broad search strategy, duplicate citation screening and abstraction, inclusion of diverse acute care trials before and after SAGER guideline publication, adjudication of SGBA with a statistician and scrutiny of appendices of included trials for SGBA.4 Our study also has limitations. First, we only examined trials published in selected journals and years that frequently publish landmark acute care trials. The decision not to include subspecialty journals (eg, cardiology) may have resulted in a lower number of acute care cardiology trials. However, this approach enabled us to sample trials from journals with high standards for publication before and after SAGER guideline publication. Second, our search was conducted solely with the MEDLINE database, and thus theoretically could miss citations, however, the risk of missing citations is low given we only focused on very high-impact journals. Third, the period after SAGER guideline publication may not have been long enough to permit guideline adoption, and we did not capture the date of individual trial registration or conduct. However, awareness of the importance of SGBA dates back to at least 2007.13 Notwithstanding, current guidance documents pertaining to conduct of subgroup analyses recommend that they be conceptualised a priori, hypothesis generating and limited to those with biological plausibility to minimise the risk of false-positives.38 39 Approaches to the conduct of SGBA were not addressed in these trials. Fourth, trials may feature substudies published after the parent trial which specifically address SGBA and diversity metric reporting not examined in the initial publication. Our findings, therefore, may under-represent SGBA reporting as we did not search for such substudies. However, the ideal time to report SGBA and diversity metrics in participant demographics would be within the initial trial publication or in an accompanying supplement given the impact this has on trial generalisability, while substudies can feature more detailed analyses and discussion. Fifth, we did not consider sex-specific disease prevalence or evaluate power issues related to SGBA. Sixth, we restricted studies to those with adult participants. Finally, we did not assess trial risk of bias as our goal was to characterise SGBA and diversity reporting.
SGBAs are important as they identify potential differences between sexes or genders in pharmacokinetic and/or pharmacodynamic effects of interventions, pathophysiology, presentation and disease course.40 Similar to other subgroup analyses, SGBAs are subject to limitations of power, potentially resulting in false negatives or false positives related to multiple comparisons.41 42 Therefore, trialists may be dissuaded from conducting an SGBA without a strong rationale.43 44 At a minimum, SGBAs are hypothesis generating and permit pooling of sex or gender-disaggregated data in subsequent meta-analyses. Prior reviews in cardiovascular disease found that one-third of trials conducted stratified analyses by sex or gender and noted that SGBA reporting increased over time.43 45–47 Conversely, we found that only 26% of acute care trials reported SGBA with no temporal improvement in SGBA reporting. Similar to other cardiology and neurology reviews, we identified that few trials reported SGBAs with a test for interaction.20 45 46 48–52
Our review is novel in examining SGBA reporting, and the discordant use of sex and gender terminology within baseline characteristics tables and between these tables and SGBAs. A review of 75 state and federal databases in the USA found that 49% of databases used gender and sex terminology inappropriately, often conflating the terms. Only 8/38 (21.2%) databases provided additional, non-binary, gender classifications.53 Accurate reporting of disaggregated sex and gender data is necessary as a precursor to the conduct of SGBA. Conflation of these variables in reporting participant characteristics, conducting analyses and interpreting findings, is likely to overstate the generalisability of findings and miss opportunities to identify the impact of these characteristics, alone or in intersection with other factors, on outcomes. Additionally, we noted that acute care trials enrol nearly 80% white participants, two-thirds of whom are males/men. We also found that race and ethnicity were heterogeneously reported using various classification systems. Poor race and ethnicity reporting were compounded by incomplete or missing data and legislation in some countries that prohibits collection of data related to participant race and ethnicity.54 The under-representation of racial minorities in acute care trials impairs the generalisability of findings to clinical practice.10 55
Similar to others, we found that females/women (vs males/men) were under-represented in acute care trials.12 56 Additionally, we identified that representation of females/women in acute care trials did not improve over time. A review of author guidance documents from 190 academic journals found that only 24% of journals explicitly distinguished between or defined the terms sex and gender, and only 34% had a policy for reporting sex or gender.57 Under-representation is important because it limits generalisability of findings and may exacerbate existing sex-based and gender-based disparities in healthcare including access to potentially beneficial interventions. In turn, this limits the conduct of sex-specific analyses and opportunities to tailor therapies to specific participant groups. Of recent concern is the effect that the under-representation of participants of various sex, gender identity, race, ethnicity and other diversity metrics, may have on the development and implementation of artificial intelligence and machine learning algorithms.58 The reasons for lower representation of females/women in trials are multifactorial. Studies suggest that the diagnosis, treatment and outcomes of females/women (vs males/men) differ based on sex and gender-disease prevalence and presenting symptomology.7 Fowler et al reported that despite males/men and females/women having similar disease severity at ICU admission, females/women over 50 years were less likely than males/men to be admitted to ICU and receive life-prolonging measures.59 Similar findings have been reported in cardiology, where cardiovascular risk is often underestimated in females/women resulting in a lower referral rates for interventions including percutaneous coronary intervention for acute coronary syndrome12 and worse outcomes including mortality.59 60 Referral biases limit opportunities for females/women to be approached for and included in clinical trials.7 This compounds the fact that females/women less frequently meet eligibility criteria due to comorbidities that vary in prevalence by sex and gender. The impact of gendered-social factors, cultural and socioeconomic influences on trial eligibility remains poorly characterised.
Conclusion
Our findings highlight a strong need for improved reporting of SGBA, diversity metrics and female/women representation in acute care trials.61 Efforts to educate researchers about the importance of these metrics as determinants of health, and enhance collection and reporting of sex, gender, and other diversity metrics are needed. Standardised and mandatory reporting requirements by funding agencies and journals may facilitate adherence to the PROGRESS PLUS62 and SAGER reporting frameworks.4
Data availability statement
Data are available on reasonable request. Complete statistical analysis plan and raw data are available on author request.
Ethics statements
Patient consent for publication
Ethics approval
Given the nature of the study no individual patient level data was used. All data was obtained from published clinical trials. No specific ethics approval was obtained.
Acknowledgments
We thank David Lightfoot for their help with the development and implementation of our literature search.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @MyancaRodrigues, @khome, @fwd_going
Contributors DG and KEAB, as guarantors, had full access to all the data in the study and take responsibility for the integrity of the overall content including data and the accuracy of the data analysis. The corresponding author (KEAB) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. KEAB conceived the study idea. DL performed the literature search. DG, MR, VR, KH, JOF, LT, LP, KEAB contributed to study design. DG, AA, KH, JOF and KEAB participated in study screening. DG, AA, KH, JOF, BP, RS, VF, GR, MK, SV, DC, CG-B, FD'A, DW, VIL, CL, JR, VT, VP, EB-C, MAIM, KEAB performed data abstraction. MR, LT, KEAB and DG helped develop the statistical analysis plan, which was performed by MR. DG, KEAB, MR, KH and VR all contributed to writing of the manuscript, however, all authors reviewed the results and contributed to editing of the manuscript.
Funding KEAB holds a Physician Services Incorporated Mid-Career Research Award and an American Thoracic Society Recognition Award for Scientific Achievement. CL holds an AFP Clinician Educator Early Career Award at McMaster. EB-C holds a National New Investigator Award from the Heart and Stroke Foundation of Canada. FD'A is supported by grants from both CIHR as well as the Fond de recherche du Quebec-Sante. JR holds a Canadian Institutes of Health Research Health Systems Impact Post-Doctoral Fellowship award. MK is funded by a Canada Research Chair in Critical Care Rehabilitation and Knowledge Translation. KH holds a Critical Care Trials Group trainee travel award (2019/2020) which was unrelated to the current manuscript. JR is supported by a CIHR Health Systems Impact Fellowship which was unrelated to the current work. CG-B receives salary support from the Ministry of Health and Long-Term Care-Clinician Investigator Program and the Department of Anesthesia and Pain Medicine at the University of Toronto. MR is supported by the Ontario Graduate Scholarship (OGS; 2021-2023) and the Research Institute of St. Joseph’s Studentship Award (2023-2024). There was no other specific funding for this manuscript and none of the above funders had any role in the design, data analysis and interpretation, writing of the report or decision to publish.
Competing interests All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/. EB-C has received investigator-initiated grant funding from Bayer, BMS-Pfizer and Roche Diagnostics and consulting honoraria from Trimedic Therapeutics, which were unrelated to the current work. JR is a co-methodologist on the Society of Critical Care Medicine Guideline Committee–End of Life Care in the ICU Guidelines. VP is a member of the Canadian Critical Care Society–Equity, Diversity, Decolonisation and Inclusion Committee. KEAB is President of the Canadian Critical Care Society and an Ex-officio member of the Canadian Critical Care Trials Group Executive Committee. There are no other relationships or activities that could appear to have influenced the submitted work.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.