Article Text
Abstract
Objective To examine longitudinal trends in clinical management of lactational mastitis in women attending general practice.
Design Open cohort study.
Setting Australian general practice using data from MedicineInsight.
Participants Women aged 18 to 44 years with one or more clinical encounters for lactational mastitis between January 2011 and July 2022.
Primary and secondary outcome measures The primary outcome measure was the proportion of prescribed oral antibiotics based on the antibiotic type. Secondary outcome measures were the proportion of women prescribed other medications (eg, antifungals, lactation suppressants) or ordered selected clinical investigations including breast ultrasound, blood test, breast milk culture, nipple swab culture or breast aspirate. Outcomes were examined based on the calendar year and individual- or clinical practice-level characteristics.
Results Among 25 002 women who had one or more clinical encounters related to mastitis, 90.9% were prescribed oral antibiotics. While the proportion of women prescribed an oral antibiotic remained consistent from 2011 to 2022 (91.1% vs 92.5%), there were changes in the proportion receiving prescriptions for di/flucloxacillin (46.1% vs 60.4%) and cefalexin (38.6% vs 26.5%). Fewer than 12% of women were clinically investigated for their mastitis encounter, most commonly a breast ultrasound (7.1%), followed by a selected blood test (3.8%). Requests for breast milk cultures, nipple swab cultures or breast aspirates occurred in less than 1.1% of individuals. Significant increases were evident with respect to ordering of all clinical investigations, with rates at least doubling between 2011 and 2022 (6.6% vs 14.7%). Large variability in clinical management was evident according to both individual- (eg, concessional status) and clinical practice-level characteristics (eg, remoteness).
Conclusions Australian general practitioners commonly prescribe oral antibiotics to women with mastitis and largely in line with clinical guidelines. Their use of clinical investigations as part of mastitis management has increased over the last decade.
- Epidemiology
- Primary Care
- Public health
- MICROBIOLOGY
- Pharmacology
Data availability statement
Data may be obtained from a third party and are not publicly available. Data may be obtained from MedicineInsight and are not publicly available. Third parties may express an interest in the information collected through MedicineInsight. The provision of information in these instances undergoes a formal approval process and is guided by the MedicineInsight independent external Data Governance Committee. This Committee includes general practitioners, consumer advocates, privacy experts and researchers.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The study includes information about >25 000 lactational mastitis encounters extracted from electronic medical records in Australian general practice, which are nationally representative.
We explored longitudinal changes in the frequency of oral antibiotic prescription, selected requested tests related to mastitis encounters and examined how these differed according to patient- and practice-level characteristics.
The quality and accuracy of ‘real-world’ data captured through electronic medical records might be affected by clinician behaviours, types of health information systems used in each general practice and algorithms used for data extraction.
Introduction
Lactational mastitis is a common breastfeeding complication, characterised by localised breast pain, tenderness, erythema and engorgement and systemic symptoms such as fever, accompanied by malaise and rigours.1 2 Mastitis can significantly disrupt activities of daily living and is associated with significant maternal morbidity1 2 and premature breastfeeding cessation.3 Mastitis prevalence ranges from 3% to 20%4 and most commonly occurs within the first 4 weeks postpartum.5 Diagnosis is based on symptoms, which range from mild inflammation to more severe disease including bacterial infection and abscess development.6 7 Early and appropriate treatment of mastitis is important to prevent adverse sequelae.
Early or mild cases of mastitis are treated symptomatically, with the use of self-management strategies aimed at ensuring effective drainage of breast milk, such as continued regular breastfeeding and/or expressing, gently massaging the affected breast, applying warmth to assist with let-down reflex and cold to reduce swelling.6 Analgesics and anti-inflammatories may be useful in the management of pain and/or fevers.8 In cases where symptoms do not resolve within 24–48 hours or are moderate or severe, treatment with antibiotics may be required.8 In Australia, the Therapeutic Guidelines advise initial empirical treatment with narrow-spectrum di/flucloxacillin,9 targeting the most likely pathogens associated with mastitis, including Staphylococcus aureus.5 10 In the case of penicillin allergy, cefalexin or clindamycin may be used, depending on the severity of the penicillin allergy.9 In contrast to antibiotics, there is less guidance regarding the use of clinical investigations as part of mastitis management. The use of breast milk or nipple swab cultures is only recommended for patients with sepsis or who are not responding to first-line treatment.6 11 Similarly, diagnostic ultrasound of the breast is recommended when a fluctuant breast mass is present, mastitis is not resolving or an abscess is suspected.6 11 Less clear is the role of blood tests such as C-reactive protein (CRP) to guide antibiotic treatment.12 13
There are few studies examining the clinical management of mastitis, particularly in a primary care or community setting. Most studies have focused on prescribing oral antibiotics, with prevalence ranging from 38% to 86%.14–19 In contrast, there has been limited exploration of clinical investigations such as ultrasound, breast milk or swab cultures. Foxman et al appears to be the only study assessing the prevalence of culture analysis, with no participants reporting having this performed.14
Given the lack of research internationally on the clinical management of lactational mastitis in general practice settings, this study aimed to investigate longitudinal trends in the clinical management of lactational mastitis among women attending Australian general practice between 2011 and 2022.
Methods
Ethics
The independent MedicineInsight Data Governance Committee approved the study (protocol 2019–003), and the Human Research Ethics Committee of the University of Adelaide exempted it from ethical review due to the use of non-identifiable data.
Study design, setting and data source
This was an open cohort study using data from the NPS MedicineWise MedicineInsight dataset. The study period spanned from 1 January 2011 to 31 July 2022. MedicineInsight is a large-scale, national general practice dataset established by NPS MedicineWise with core funding from the Australian Government Department of Health. The MedicineInsight dataset has been described in detail elsewhere.20 In summary, MedicineInsight uses third-party extraction tools (GRHANITETM and Precedence Healthcare’s cdmNetTM) to extract, de-identify and securely transmit patient data from participating practices’ clinical information systems, such as Best Practice and Medical Director, to a secure data repository. The extraction tool collects incremental data regularly, allowing the development of a longitudinal database in which individuals within each practice can be tracked over time. The MedicineInsight dataset collects data on individual demographic characteristics, practice encounters (not including progress notes), diagnoses, prescribed medication, pathology tests and referrals. Insights are enriched through selected free-text data. MedicineInsight contains electronic health records from approximately 2700 general practitioners (GPs) and 662 general practices across Australia (8.2% of all Australian practices).20 The characteristics of MedicineInsight patients have been previously demonstrated to be nationally representative of the Australian population.20
Study population
We restricted our analysis to females of reproductive age (18–44 years inclusive) with one or more documented clinical encounters related to mastitis and documentation relating to pregnancy within the previous 12 months of the encounter. Mastitis encounters were identified by searching the ‘Encounter reason’ free-text field for the term ‘mastitis’. We also searched the ‘Diagnosis reason’, ‘Test reason’ and ‘Prescription reason’ free-text fields for the term ‘mastitis’ to identify encounters related to mastitis. We excluded the free-text term ‘granulomatous mastitis’ as this was considered unlikely to be related to lactational mastitis. Clinical encounters for mastitis occurring within 14 days of a previous mastitis encounter were defined as belonging to the same treatment episode. Only the first episode per individual was included in the analysis. Documented pregnancies were identified using the separate ‘pregnancy’ dataset, which included data on the date of the last menstrual period and the estimated date of confinement. We also searched the ‘Encounter reason’ free-text field using terms related to pregnancy (ie, ‘Antenatal’, ‘Pregnancy’, ‘Hyperemesis gravidarum’, ‘Morning sickness’), postpartum (‘postnatal’, ‘postpartum’, ‘baby check’, ‘6-week check’) or breastfeeding (ie, ‘breast feeding’, ‘breastfeeding’, ‘lactation’) to identify women with a recent pregnancy. This was undertaken to increase the likelihood of the clinical encounter being related to lactational mastitis.
Outcome
The primary outcome assessed was the proportion of women prescribed oral antibiotics on the same date as a mastitis encounter. Prescribed antibiotics were identified from the corresponding ‘Prescriptions’ dataset. We extracted data on the antibiotic type, quantity supplied and whether any repeat prescriptions (for subsequent medication supplies) were issued. Secondary outcomes included the proportion of women ordered clinical investigations for mastitis including breast ultrasound, breast milk culture, nipple swab culture, blood test (ie, CRP, erythrocyte sedimentation rate, full blood examination) and breast aspirate. These were identified by searching the ‘Requested tests’ free-text field for the previously listed terms. Additional secondary outcomes included the proportion of women prescribed other medications, including topical or intravenous antibiotics, antifungals, lactation suppressants (ie, cabergoline, bromocriptine) or lactation stimulants (ie, domperidone).
Patient and public involvement
There was no direct patient or public involvement in this research. The research used an established de-identified general practice dataset.
Covariates
Patient characteristics included age (based on year of birth), remoteness, socio-economic indexes for areas (SEIFA), state/territory, Indigenous status, Commonwealth concession card status and smoking status. Females for whom Indigenous status was recorded as unknown or missing were re-categorised as non-Indigenous, as done in other studies.21 Remoteness, SEIFA and state/territory were based on patients’ residential postcodes. Remoteness was determined in accordance with the Australian Bureau of Statistics (ABS)’s Australian Statistical Geography Standard Remoteness Areas, with one being a ‘Major City’ and being in a ‘Very Remote’ area. Due to small population sizes, data for ‘Remote’ and ‘Very Remote’ were combined. SEIFA was determined according to the ABS Index of Relative Socio-Economic Advantage and Disadvantage codes. We also extracted data from the clinical observation dataset to determine which individuals had a temperature recorded on the same day as the clinical encounter and whether the patient was considered febrile or not (temperature >38.5°C).
Statistical analysis
Descriptive statistics (counts and percentages) were used to describe the study population.
The proportion of women who were prescribed medications or ordered selected clinical investigations was calculated based on the year of first clinical encounter for mastitis and expressed as a percentage, with corresponding 95% CIs. Proportions were calculated separately based on management occurring on the same day as the first documented clinical encounter for mastitis or on the same day as any clinical encounter for mastitis within the same episode. The proportion of women prescribed antibiotics or who received selected clinical investigations was stratified by calendar year to examine longitudinal trends.
We examined practice-level variation in the proportion of women receiving selected management for mastitis by stratifying proportions based on individual general practices, restricting the comparison to general practices that included data on ≥10 patients with mastitis.
We used univariable logistic regression analyses to compare the likelihood of women being prescribed oral antibiotics or receiving various clinical investigations related to mastitis based on individual characteristics. These analyses were undertaken separately according to clinical management at the first encounter or any clinical encounter within the same mastitis episode.
All analyses were based on two-sided P values, which were statistically defined by p<0.05. The statistical analysis was performed using STATA MP 17 (Stata, College Station, Texas), with graphs prepared using R version 4.3.0 (R Core Team).
Results
A total of 25 002 females aged 18 to 44 years had one or more clinical encounters related to mastitis recorded in this general practice dataset between January 2011 and July 2022, as well as documented evidence of a recent pregnancy.
A greater proportion of women were aged 30–34 (39.3%), were never smokers (54.6%), lived in a major city (65.6%) and had a very high socio-economic status (27.5%). A small proportion of women held a Commonwealth Concession card (15.6%) or were Aboriginal and/or Torres Strait Islander (TSI) (2.2%) (table 1). Approximately one quarter (27.8%) of women had a documented temperature at their first encounter, with 5.8% of those with a documented temperature being febrile.
Characteristics of 25 002 women presenting to the Australian General Practice between 2011 and 2022 for Lactational Mastitis
Most (90.1%; n=22 523) women received a prescription for oral antibiotics at their first encounter. With respect to clinical investigations, 5.6% were ordered a breast ultrasound, 3.3% a blood test, 0.9% a nipple swab culture and 0.8% breast milk culture (table 2). Only very small numbers were prescribed oral (1.1%) or topical (1.2%) antifungals, topical antibiotics (0.5%), lactation suppressants (1.1%) or lactation stimulants (1.0%). Di/flucloxacillin and cefalexin accounted for >90% of oral antibiotic prescriptions. Only 3006 (12.0%) women had two or more clinical encounters related to the same mastitis episode. When including clinical management across all clinical encounters, the proportion of prescribed oral antibiotics increased only marginally to 90.9%. When considering investigations ordered at any clinical encounter, the largest increase was observed for breast ultrasound, which increased from 5.6% to 7.1% (table 2). Approximately 1 in 10 (12.1%) women prescribed cefalexin had a documented penicillin allergy, whereas 64.4% of women prescribed clindamycin had a documented penicillin allergy.
Selected clinical investigations and medications prescribed during the first or any encounter within the same episode of treatment for lactational mastitis in women attending general practice, Australia, 2011 to 2022
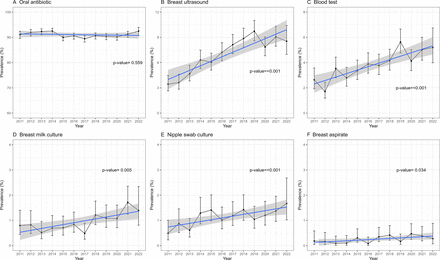
When stratified by calendar year, the proportion of women prescribed oral antibiotics remained consistent from 2011 to 2022 (p=0.559). In contrast, significant increases from 2011 to 2022 were evident with respect to increases in proportions undergoing breast ultrasound (3.4% to 8.5%), blood test (2.6% to 5.2%), breast milk culture (0.8% to 1.4%), swab culture (0.5% to 1.7%) and breast aspirate (<0.1% to 0.4%) (figure 1, online supplemental table 1). The proportion of women prescribed di/flucloxacillin increased from 46.1% in 2011 to 60.4% in 2022, whereas the proportion prescribed cefalexin decreased from 38.6% to 26.5% (online supplemental table 2). From 2011 to 2022, the median treatment duration based on the initial prescription was 6 days; however, there was a significant reduction in the proportion of women issued repeats from 31.5% to 3.0%.
Supplemental material
Longitudinal trends in the proportion of women attending the general practice for lactational mastitis who are prescribed oral antibiotics or undergo selected clinical investigations, Australia 2011 to 2022.
Significant variability was evident with respect to the overall clinical management of mastitis according to individual general practices, with the proportion of patients prescribed oral antibiotics ranging from 56.9% to 100% (figure 2). Likewise, variation was seen with breast ultrasound (range: 0% to 40.6%), breast milk culture (0% to 14.7%), swab culture (0% to 9.2%), breast aspirate (0% to 3.8%) and blood test (0% to 31.2%).
Variation in the proportion of women attending general practice for lactational mastitis who are prescribed oral antibiotics or undergo selected clinical investigations according to each general practice, Australia 2011 to 2022. Each circle corresponds to an individual site.
Women prescribed oral antibiotics at the first encounter were less likely to receive a breast ultrasound, blood test and breast aspirate. Similarly, they were less likely to be co-prescribed topical antibiotics and oral/topical antifungals (online supplemental table 3).
The only consistent factor associated with an increased likelihood of being prescribed oral antibiotics at the first or any encounter was the general practice being located in a regional or remote area, but the absolute differences were small (~1.0%) (table 3). In comparison, factors associated with an increased likelihood of breast ultrasound included older age (>30 years), being a current smoker, living in a major city and having a higher socioeconomic status (online supplemental table 4). The likelihood of receiving a blood test was increased for those in the youngest (18–24 years) and oldest (40–44 years) age groups, with a concession card (online supplemental table 5). Factors associated with an increased likelihood of a breast milk culture included having a concession card, being a previous or current smoker, and the general practice being located in a regional or remote area (online supplemental table 6). In contrast, the only factor associated with an increased likelihood of receiving a nipple swab culture was the general practice being located in a regional or remote area (online supplemental table 7). In addition, however, the likelihood of receiving a breast milk culture was lower for those with a concession card or who were previous or current smokers. Only lower socio-economic status was associated with a lower likelihood of breast aspirate (online supplemental table 8), although none were ordered for individuals identified as Aboriginal and/or TSIs or who were identified as being febrile at the time of the first encounter.
Proportion of women attending general practice who were prescribed oral antibiotics during the first, or any, encounter for lactational mastitis according to individual- and practice-level characteristics, Australia 2011 to 2022
Discussion
Principal findings
Evidence from this large national database indicates that most women presenting to Australian general practice for lactational mastitis are prescribed oral antibiotics, with prescribing practices appearing to largely be in adherence to Australian clinical guidelines. While there has been no change in the overall oral antibiotic prescribing rates over the past decade, we observed an increase in prescribing of narrow-spectrum antibiotics (ie, di/flucloxacillin). This, combined with a lower rate of repeat prescription orders over time, indicates closer adherence to local guidelines and improved antibiotic stewardship. In addition, there were significant increases in the use of selected clinical investigations as part of mastitis management, including breast ultrasound and milk and nipple swab cultures. This suggests increased awareness of the use of clinical investigations in supporting optimal clinical management of lactational mastitis. The observed variation in the clinical management of lactational mastitis according to patient- and practice-level characteristics warrants further investigation to determine whether there may be further opportunities to improve the standardisation of clinical care.
Strengths and weaknesses
Study strengths include the use of a large high-quality general practice database containing longitudinal individual-level data from 2011 to 2022 that is considered nationally representative. Multiple strategies were used to improve data quality, including restricting the cohort to those with a likely recent pregnancy and the use of different fields for data extraction. Nonetheless, our study has some important limitations. The quality and accuracy of ‘real-world’ data captured through electronic medical records might be affected by clinician’s behaviours and the type of health information system used in each general practice. For example, differences in recording may exist based on non-mandatory fields, free-text entries and use of system coding vocabularies. It is possible that this may result in possible misclassification and over- or under-reporting of lactational mastitis encounters or associated clinical management. As the study team was only provided access to de-identified data, we were unable to perform chart reviews to validate our approach for identifying women diagnosed with lactational mastitis. Further, we assumed that prescriptions or clinical investigations ordered on the same day as a clinical encounter for mastitis were related to that encounter reason, where they may have been provided for alternative indications. Also, given clinical investigations may have been undertaken to rule out diagnoses of lactational mastitis, it is possible that individuals identified to have undergone clinical investigations may be more likely to have been misclassified as having lactational mastitis. In addition, GPs commonly provide a prescription for antibiotics (or other medications) with directions only to get it dispensed if symptoms don’t improve in the subsequent days (‘delayed prescribing’).22 The database doesn’t contain information on referrals made to other healthcare providers or hospitals. In the case of severe mastitis, some clinicians may have opted to direct the patient to a hospital emergency department rather than provide treatment. While data are recorded at the individual patient level, patient data are not linked across different general practices. Therefore, it is possible that individuals presenting to different general practices with the same symptoms were counted twice. Lastly, MedicineInsight uses a non-random sampling process to recruit the practices; however, the sample distribution has previously been shown to closely resemble figures from the last Australian census.20
Comparison to other studies
To our knowledge, this is the first evaluation of clinical management of lactational mastitis in a primary care setting. Most previous studies are limited to small numbers of women with mastitis (often less than 100) and lack contemporary data. In a prospective cohort study, Foxman et al followed 946 women recruited between 1994 and 1998, of whom 77 developed mastitis.14 Of these women, 86% were prescribed antibiotics, with cefalexin (46%) being the most common, followed by amoxicillin (7%), ampicillin (7%) and amoxicillin and clavulanic acid (7%). No cultures were performed for any women, and no data were reported for ultrasound or blood tests. A more recent study by Scott et al followed 420 breastfeeding women recruited between 2004 and 2005 in Scotland, with 74 developing mastitis.19 Among those women with mastitis, 78% were prescribed antibiotics, with flucloxacillin (30%) being the most common, followed by amoxicillin (17%), erythromycin (7%) and amoxicillin and clavulanic acid (7%). Notably, 33% couldn’t remember what antibiotic they were prescribed. The largest study evaluated data from almost 80 000 women recruited to the Norwegian Mother, Father and Child Cohort Study between 1999 and 2008.3 Among the 15 014 who reported experiencing mastitis, 5524 (36.8%) reported using antibiotics. Of those who could remember which antibiotic they received, the most common antibiotics included penicillins (83.8%), followed by macrolides (15.2%) and cephalosporins (2.6%).
Implications
While the observed proportion of antibiotic prescribing is very high, it is not possible to determine the appropriateness of prescribing practices based on available data. It is possible that women presenting to general practice with mastitis symptoms may represent those with more severe disease, corresponding to high rates of treatment. Further, it is likely that not all prescriptions for antibiotics were dispensed, and even when dispensed, not all antibiotics were commenced.23 Also, it is possible that GPs are issuing prescriptions (or repeats) to women to be dispensed only if symptoms do not improve, representing delayed prescribing. However, the role of delayed prescribing in the context of antimicrobial stewardship for mastitis has not been evaluated and requires further investigation. Further, there was a much larger observed variation in antibiotic prescribing practices at the practice-level compared with patient-level characteristics, and this warrants further exploration of the underlying causes.
Increasing rates of the ordering of clinical investigations over the decade suggest increased awareness of the potential value of such investigations in the diagnosis and/or management of mastitis. The observed higher frequency of ultrasound in older women and current smokers suggests ultrasounds may be used to rule out differential diagnoses.24 25 However, lower rates of ultrasound in those of lower socio-economic status raises questions about potential equity issues and whether access or affordability negatively impacts the uptake. A similar picture emerged for other investigations such as breast aspirate, breast milk culture and nipple swab culture, which were lower in those with lower socio-economic status or a concession card. The lower rate of ultrasounds in those visiting a general practice located in remote or regional areas may be reflective of access difficulties; however, this is contrasted with higher rates of milk and swab cultures in these areas. Higher culture rates in rural settings may reflect observed higher rates of antimicrobial resistance, including Methicillin-resistant S. aureus (MRSA) in these settings.26 Similar to antibiotic prescribing practices, the large variation in the frequency of clinical investigations warrants further examination, particularly the appropriateness of these investigations and their impact on mastitis management.
Lactational mastitis has frequently been considered to be a topic that has not received the attention it deserves.27 Research is needed to determine when antibiotics are needed for mastitis, which is the most appropriate antibiotic and what is the most appropriate duration. Clinical guidelines for the management of mastitis have changed little over the last couple of decades, and GPs are assumed to be familiar with managing this common problem. It is timely to recommend an educational programme to alert clinicians that prescribing antibiotics for mastitis is not always straightforward: it should not be assumed that all lactating women with inflammatory breast symptoms require antibiotic treatment, and the potential need for breast milk culture should be considered. Breast milk culture may be appropriate at the first presentation in locations with high rates of MRSA or mothers with known antibiotic allergies and should be ordered if the condition is not responding to first-line antibiotics within 48 hours.8
Conclusion
Australian GPs commonly prescribe oral antibiotics for women with mastitis and largely in line with clinical guidelines. Over the last decade, GPs have increased prescriptions for narrow-spectrum antibiotics indicating closer adherence to local guidelines and improved antibiotic stewardship. Their use of clinical investigations as part of mastitis management has increased over the last decade, but there may be the opportunity for increased use of breast milk culture to understand and manage local bacterial sensitivities.
Supplemental material
Data availability statement
Data may be obtained from a third party and are not publicly available. Data may be obtained from MedicineInsight and are not publicly available. Third parties may express an interest in the information collected through MedicineInsight. The provision of information in these instances undergoes a formal approval process and is guided by the MedicineInsight independent external Data Governance Committee. This Committee includes general practitioners, consumer advocates, privacy experts and researchers.
Ethics statements
Patient consent for publication
Ethics approval
The independent MedicineInsight Data Governance Committee approved the study (protocol 2019-003), and the Human Research Ethics Committee of the University of Adelaide exempted it from ethical review due to the use of non-identifiable data.
Acknowledgments
The authors would like to thank NPS MedicineWise for their support in the development of this research.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @LukeGrzeskowiak, @Lisa_H_Amir
Contributors All authors made significant contributions to the manuscript and are responsible for its content. LEG, SBC, MC and LHA conceived the idea, obtained grant funding and planned this study. LEG and AK were responsible for data extraction and analysis. LEG, SBC, MC and LHA were responsible for the interpretation of study findings, while LEG and AK were responsible for presenting the results. LEG wrote the first draft, and all authors contributed to the manuscript refinement. All authors have read and approved the final manuscript. LEG accepts responsibility for the overall content as guarantor
Funding This project was funded by a Therapeutic Guidelines Ltd (TGL)/RACGP Foundation Research Grant (TGL2020-02) awarded to LEG, SBC, MC and LHA. LEG receives salary support from Channel 7 Children’s Research Foundation (CRF-210323).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.