Article Text
Abstract
Objective To evaluate the effectiveness of standardised antiretroviral therapy (ART) among different HIV subtypes in people living with HIV/AIDS (PLWHA), and to screen the best ART regimen for this patient population.
Design A retrospective cohort study was performed, and PLWHA residing in Huzhou, China, between 2018 and 2020, were enrolled.
Setting and participants Data from 625 patients, who were newly diagnosed with HIV/AIDS in the AIDS Prevention and Control Information System in Huzhou between 2018 and 2020, were reviewed.
Analysis and outcome measures Data regarding demographic characteristics and laboratory investigation results were collected. Immune system recovery was used to assess the effectiveness of ART, and an increased percentage of CD4+ T lymphocyte counts >30% after receiving ART for >1 year was determined as immunopositive. A multiple logistic regression model was used to comprehensively quantify the association between PLWHA immunological response status and virus subtype. In addition, the joint association between different subtypes and treatment regimens on immunological response status was investigated.
Results Among 326 enrolled PLWHA with circulating recombinant forms (CRFs) CRF01_AE, CRF07_BC and other HIV/AIDS subtypes, the percentages of immunopositivity were 74.0%, 65.6% and 69.6%, respectively. According to multivariate logistic regression models, there was no difference in the immunological response between patients with CRF01_AE, CRF07_BC and other subtypes of HIV/AIDS who underwent ART (CRF07_BC: adjusted OR (aOR) (95% CI) = 0.8 (0.4 to 1.4); other subtypes: aOR (95% CI) = 1.2 (0.6 to 2.3)). There was no evidence of an obvious joint association between HIV subtypes and ART regimens on immunological response.
Conclusions Standardised ART was beneficial to all PLWHA, regardless of HIV subtypes, although it was more effective, to some extent, in PLWHA with CRF01_AE.
- HIV & AIDS
- preventive medicine
- public health
Data availability statement
Data sharing is not applicable as no data sets were generated and/or analysed for this study. Considering the particularity of the research object, the data of this study are not suitable for sharing.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The present study was based on a population-based cohort rather than case-control or descriptive investigations.
The study analysed the association between HIV subtypes and immunological responses, considering the joint association of subtypes and ART regimens.
HIV-1 subtypes were regionally relevant and sampling bias was inevitable.
This study had a small sample size because HIV-1 genotyping is not routinely performed in China.
Data regarding viral load was missing from a large number of records.
Introduction
AIDS is a major public health problem.1 As of 2019, 38 million individuals worldwide are living with HIV, and 690 000 have died from HIV-related illnesses.2 HIV harms human health primarily by infecting the body and destroying immune cells, and individuals living with HIV often live as asymptomatic carriers for decades or more before eventually developing AIDS and secondary comorbidities.3 In China, the HIV epidemic has generally stabilised from an annual increase at the beginning of twenty-first century.4 However, there is wide variation in the type of epidemic and geographical spread, which poses a great challenge to the prevention and control of AIDS in China.5
Although there is still no effective cure for AIDS, previous studies have shown that antiretroviral therapy (ART) is widely used to control HIV/AIDS, with good results.6 Since 2016, all people living with HIV/AIDS (PLWHA) in China, regardless of their initial CD4+ T lymphocyte (CD4) cell levels, have been eligible to receive free-state ART and regular follow-up visits by health service staff.7 In 2019, the world has entered an era of universal access to ART, and many developing countries, including China, have in large part achieved full coverage of ART, with an increasing number of PLWHA receiving free ART and achieving viral control.8 However, the HIV epidemic in China has shown no signs of slowing down.9
HIV, a highly diverse virus, exhibits significant genetic variability and a high viral replication rate, which may produce biological variability that affects treatment outcomes.10 There are two major HIV types—HIV type 1 (HIV-1) and HIV type 2 (HIV-2), and PLWHA with HIV-2 tend to have a lower viral load (VL) than those with HIV-1. HIV-1 is by far the most prevalent type, with almost 95% of global HIV infections of type 1, and HIV-1 is further divided into four groups, including groups M, O, N and P. Group M is the world’s major epidemic pathogen of HIV,11 and is further divided into 10 subtypes (A, B, C, D, F, G, F, J, K and L), a series of circulating recombinant forms (CRFs)12 and unique recombinant forms. Currently, CRFs formed by recombination among subtypes B, C and CRF01_AE are the most common in China. HIV-1 CRF01_AE and CRF07_BC account for 36.2% and 40.8% of the population reported to be infected in 2018, respectively, according to the results of the 2018 China Molecular Epidemiology Survey,13 and these two branches have become the most predominant CRFs of HIV in China.14 CRF01_AE was reported to harbour a high prevalence of CXCR4 viruses,15 which contributed to rapid CD4 count depletion in natural infection16 17 and suboptimal CD4 restoration during ART.18 Studies investigating the effectiveness of ART in patients with major HIV-1 subtypes have been inconclusive. An analysis of the impact of HIV-1 subtype diversity on long-term clinical outcomes of ART in Guangxi Province suggested that patients with CRF01_AE may benefit more from immediate ART than those with CRF07_BC.19 However, studies in southern Nigeria and the UK did not report an association between HIV-1 subtypes and immunological or virological responses after treatment.20 21 Therefore, we hypothesised that the effectiveness of ART differs among HIV-1 subtypes. Accordingly, we aimed to analyse the association between patients with HIV/AIDS with different subtypes and immunological responses after ART in Huzhou, China, between 2018 and 2021, and to further explore whether different ART regimens have a modifying effect on the association between different HIV subtypes and immunological responses.
Methods
Study design and participants
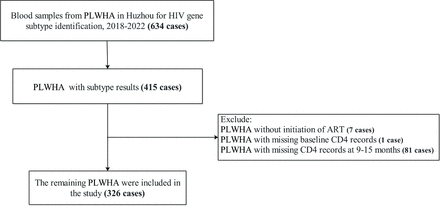
The present investigation was a retrospective cohort study. Data from 625 patients, who were newly diagnosed with HIV/AIDS in the AIDS Prevention and Control Information System (the AIDS-PCIS) in Huzhou between 2018 and 2020, were reviewed. All participants identified in the AIDS-PCIS receive a combination antiretroviral regimen containing at least three antiretroviral drugs and sign an informed consent form at the time of initiation of ART, allowing the use of clinical records in future epidemiological studies. The inclusion criteria for the study were as follows: (1) Complete laboratory blood tests before receiving ART; (2) Living in Huzhou area including temporary residents; (3) Starting ART between 1 January 2018 and 31 December 2020; (4) Having a complete record of CD4 cell counts to 9–15 months after receiving ART. Ultimately, data from 326 PLWHA were included in the present study.
Demographic characteristics and laboratory information
Data on the demographic and clinical characteristics of the study participants were collected at the time of their registration in a face-to-face survey interview or extracted from their medical records using a structured questionnaire designed specifically for AIDS-PCIS. Information collected included age, sex, height, weight, marital status, occupation, history of sexually transmitted infections (STIs), disease status, sample source, clinical staging by the WHO and route of infection. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Information on laboratory tests was obtained from the Huzhou Center for Disease Control and Prevention (CDC) or a local hospital. Tests included CD4, CD8+ T lymphocytes (CD8), VL, white blood cells (WBCs), platelets, haemoglobin, serum creatinine, triglyceride, total cholesterol, fasting plasma glucose, alanine aminotransferase, aspartate aminotransferase and total bilirubin. All laboratory parameters were assessed at the local hospital or central laboratory of the Huzhou CDC by trained technicians in strict accordance with clinical guidelines.
Study outcomes
Immunological response was defined as an increase in CD4 cell counts >30% from baseline at 12 months after initiation of ART. For those patients who did not undergo CD4 cell count testing 12 months after starting ART treatment, test results obtained at 9–15 months were selected for analysis, which was the closest estimate to 12 months.22
Statistical analysis
Because missing values introduced some bias in the results, all variables with missing ratios >30% were eliminated from the final working data set. Otherwise, the missing values were filled using a fivefold multiple imputation approach. Subsequently, a sensitivity analysis of the comparison of preimputation and postimputation was additionally applied to validate the stability of the imputations (see online supplemental table S1).
Supplemental material
We described continuous variables using mean±SD or median and IQR, and Student’s t-test or Wilcoxon rank-sum test was used to compare the differences of patients with and without immunological responses. Categorical variables were shown as proportions, and χ2 or Fisher’s exact tests were used for their comparisons. The association between immunological response and participants’ characteristics was estimated using univariable logistic regression models. Multivariable logistic regression was performed including all variables that were associated with immunological response in the univariable analysis with a value of p<0.05, and the multivariate logistic regression model was used to determine the statistical association between different HIV subtypes and immunological responses, adjusting for potential confounders, including leucocyte count, CD8, infection status and time from diagnosis to treatment. Given the large heterogeneity in the other subtypes, we selected only the two main subtypes, CRF01_AE and CRF07_BC. According to the WHO definition of obesity in Asian populations,23 the BMI cut-off value was 25 kg/m2. Other numerical variables were determined based on the median values. In addition, the joint association between the HIV subtypes and ART regimens on immunological response was estimated.
Difference was with P<0.05 were considered to be statically significant. All data management and statistical analyses were performed using Stata/MP V.15.1 for windows (Stata Corp, College Station, Texas, USA) and SAS V.9.4 (SAS Institute, Cary, North Carolina, USA).
Patient and public involvement
No subjects took part in developing the research question or study design.
Results
Description of baseline characteristics
A flow chart of participants is shown in figure 1. A total of 326 PLWHA were included in the present study, with a mean follow-up of about 1 year. The distribution of HIV subtypes was as follows: CRF01_AE (n=100); CRF07_BC (n=157); other (n=69). The mean age was 41.9±15.0 years, with 20% of participants <42 years of age. The median (quartile 1, quartile 3) baseline CD4 cell and CD8 cell counts were 279.5 (175.0–382.0) and 657.75 (481.0–913.0) cells/uL, respectively. In addition, the median (quartile 1, quartile 3) baseline leucocyte counts were 5.6 (4.5–6.7)× 109/L. Characteristics of the study participants according to immunological responses are shown in table 1. Compared with patients without immunological response, those with immunological response were more likely to exhibit higher baseline CD4, CD8 and WBC levels. They also tended to have a shorter time between HIV diagnosis and treatment. In addition, the majority of the study participants were HIV-infected at the time of ART and did not transition to AIDS status. Furthermore, gender, age, marital status and history of STIs were not significantly different between the two groups.
Flow chart of people living with HIV/AIDS (PLWHA) with different subtypes in Huzhou, 2018–2020. ART, antiretroviral therapy; CD4, CD4+ T lymphocyte.
Comparison of demographic and laboratory characteristics of PLWHA in different immunological response statuses
Association between immunological response and different HIV subtypes in PLWHA
The associations between immunological responses and the different subtypes of HIV are summarised in table 2. The proportion of positive immunological responses in PLWHA with CRF01_AE was obviously higher than that in PLWHA with CRF07_BC and other subtypes (74.0% vs 65.6% vs 69.6%). In comparison to patients infected with the CRF01_AE, individuals diagnosed with CRF07_BC and other subtypes did not exhibit a significantly distinct likelihood of eliciting an immune response [(CRF07_BC: adjusted odds ratio (aOR) (95% CI) = 0.8 (0.4 to 1.4); other subtypes: aOR (95% CI) = 1.0 (0.5 to 2.0)]), after adjusting for variables including the infection status, WBC, time from diagnosis to treatment and CD8. After removing patients with CRF01_AE, there was also no significant difference in the immunological response between the two remaining subtypes (other subtypes: aOR (95% CI) = 1.2 (0.6 to 2.3)).
Association of immunological response status with viral subtypes in PLWHA
Joint association between HIV subtypes and ART regimens on immunological response
Due to the large heterogeneity of patients with other subtypes, only two other subtypes were retained. The joint associations between the two subtypes and the three ART regimens are summarised in online supplemental table S2. Among PLWHA with CRF01_AE, the effectiveness of ART for PLWHA receiving 3TC+EFV+TDF increased by 24% for those receiving 3TC+AZT+EFV (p=0.052, OR (95% CI) = 3.4 (1.0 to 11.8)). But this effect disappeared after adjusting for infection status, WBC, CD8 and time from diagnosis to treatment. None of the other associations were significant.
Discussion
The global distribution of the HIV-1 genotypes is highly heterogeneous and varies geographically.24 In addition, the distribution of HIV-1 may be related to different routes of infection25 and forms of population mobility, etc. Various aspects of disease progression, all-cause mortality, viral suppression status and immune recovery status following ART treatment in PLWHA may also be influenced by the diversity of HIV-1 genotypes. Therefore, it is important to understand the epidemiological characteristics of HIV-1 subtypes in a given region26 to enable more targeted treatment and control of the epidemics. Results of the present study demonstrated that CRF01_AE (30.7%) was the predominant HIV-1 genotype in Huzhou, followed by CRF07_BC (17.2%). This is similar to findings reported in previous studies examining HIV-1 subtype diversity in Shanghai, Jiangsu and Guangxi provinces.27 However, there are also studies reporting different results, such as a survey of HIV-1 in Yunnan Province, which found CRF08_BC to be the most common subtype.28 Previous studies have shown that the prevalence of CRF01_AE is significantly higher in the southern provinces of China.29 This is consistent with its location of Huzhou. The difference in distribution may be related to the route of transmission. In our study, CRF07_BC was the most common genotype in the population with homosexual transmission. In fact, it has been officially reported by the state that heterosexual transmission has become a major risk factor for PLWHA in China.
China has one of the highest numbers of HIV-1 genotypes, with 10 CRFs identified for the first time in this country (CRF01_AE, CRF07_BC, CRF08_BC, CRF55_01B, CRF57_BC, CRF59_01B, CRF61_BC, CRF62_BC, CRF64_BC and CRF65_cpx). A previous study by Taylor et al reported that HIV-1 subtype diversity is associated with the response to ART.30 A comprehensive assessment of the effect of HIV-1 subtype diversity on long-term clinical outcomes during ART can help inform planning recommendations. Our study found that PLWHA with CRF07_BC had significantly higher baseline CD4 cell counts than those with CRF01_AE. Previous studies reported that PLWHA with CRF01_AE experience a faster rate of CD4 cell decline and faster progression to HIV/AIDS in natural infection.31–33 This phenomenon indicates that patients with CRF01_AE may benefit more from ART. However, we did not find any differences in the immunological responses of the different subtypes among PLWHA. We hypothesise that this may be related to the short follow-up period. In the future, it will be necessary for us to continue to follow the CD4 records of this cohort to test our hypotheses.
Our study has several limitations, the first of which are its small sample size and short follow-up period. However, identification of HIV-1 genotyping is not a routine practice in testing programmes in China, and the subtyping of this group of patients was performed in a pilot study in Huzhou. Second, HIV-1 subtypes are regionally relevant and sampling bias is inevitable. Third, there was a large amount of missing data regarding VL, but we chose immunological response as the study endpoint. Although we are currently in the era of ‘total treatment’ for HIV, long-term serial CD4 cell counts are necessary to determine disease progression and changes in immune status to assess the effectiveness of treatment. Finally, although the study population was a longitudinal cohort, we were unable to determine an accurate survival time because our outcome was an immunological response, so we constructed a multifactorial logistic regression model. Nevertheless, this is the first study from East China to analyse the association between HIV subtypes and immunological responses, considering the joint association between subtypes and ART regimens. Therefore, our findings must to be validated in cohorts with larger samples sizes.
Conclusion
In summary, we found no evidence of an association between HIV-1 subtypes and immunological responses, suggesting that currently widely used antiretroviral drugs have similar effectiveness in the subtypes that predominate in the Huzhou area.
Data availability statement
Data sharing is not applicable as no data sets were generated and/or analysed for this study. Considering the particularity of the research object, the data of this study are not suitable for sharing.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. The study protocol was approved by the Ethics Committee of the Huzhou CDC (batch number HZ2021001). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank colleagues from the Huzhou CDC and Wenzhou Medical University for their hard work and contributions.
References
Footnotes
XL, YW and ZY contributed equally.
Contributors MJ, JL, XL and ZY: conceptualisation, funding acquisition, supervision, drafting and editing. YW: conceptualisation, data management, data analysis, methodology, drafting and editing. ZT, XL, ZW, FR and XZ: conducting a research and investigation process, data management, data collection. GM: conceptualisation, data management, data analysis, methodology, writing of the review and editing, supervision. All authors reviewed and approved the final manuscript. MJ
and GM are guarantors of this study.
Funding This work was supported by the Medical and Health Research Project of Zhejiang Province (2022KY369), the Huzhou Science and Technology Research Plan Project (2023GYB28), the Huzhou Medical Key Supporting Discipline (Epidemiology), and the Key Laboratory of Emergency Detection for Public Health of Huzhou.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.