Article Text
Abstract
Introduction Carpal tunnel syndrome is a common disorder affecting a substantial portion of the general population. Surgical intervention is often deemed necessary, with the median nerve release being one of the most frequent operations. Optimising all the aspects of this procedure can enhance patient satisfaction with the treatment.
Methods and analysis We aim to determine the differences in the aesthetic outcome of the scar as well as the pain experienced during the healing process between the use of absorbable and non-absorbable sutures. The primary outcome measure will be the patients’ subjective satisfaction with the aesthetic appearance of the scar 1 year after the operation. Secondary outcomes will include a similar evaluation of the aesthetics performed by a blinded outcome assessor, as well as pain experienced by the patients during the 2 weeks postoperatively. The severity and improvement of the patients’ symptoms will also be measured by a Finnish version of the Boston Carpal Tunnel Questionnaire. Costs will be evaluated for both groups. Safety of the wound closure will be followed and reported.
Ethics and dissemination This protocol was approved by the Research Ethics Committee of the Northern Savo Hospital District (2319/2021). The trial will be conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The results will be disseminated through publication in peer-reviewed journals.
Trial registration number NCT05503719.
- Hand & wrist
- Patient Reported Outcome Measures
- Patient Satisfaction
- Clinical Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Adequately long follow-up of 1 year to evaluate final aesthetic outcome of the scar.
The comparatively large sample size should grant statistically and clinically relevant and robust results if they exist.
Due to the nature of the trial, neither the participants, nor the performing physicians can be blinded to the intervention.
Introduction
Background
Carpal tunnel syndrome (CTS) is the most common type of entrapment neuropathy in the upper extremity occurring by estimate in 3.8% of the general population.1 Two non-operative treatment options with at least moderate evidence of effectiveness are splinting and corticosteroid injection.2 Glucocorticoid treatment has the adverse effect of causing further degeneration,3 and both splinting and steroid treatment have been shown to yield mixed results long term.4 5 While non-operative treatment is the primary option, invasive treatment is often necessary. According to a recent study, 77% of patients undergoing median nerve release surgery had previously received some form of non-operative treatment.6 Carpal tunnel release surgery is considered the gold standard and has been shown to give excellent results in 75% of cases.7 Another study found a significant reduction in Symptom Severity Scale for 88% of patients treated.8 Common reasons for dissatisfaction among patients include a failed diagnosis, incomplete release, iatrogenic nerve injury, scarring of the nerve and inappropriate expectations.9 Despite the high success rate, there is a notable risk of long-term complications such as a variety of problems with the healing of the scar.10
Rationale
Due to the frequent nature of the surgical treatment for CTS, there is an increasing demand for a high-quality trial with sufficient statistical power to determine the optimal wound closure method for maximising patient satisfaction and clinical outcome. A recent integrative literature review reached similar conclusions to previous studies and emphasised the need for a new high-quality randomised controlled trial (RCT) on the subject.11 It is important to note that non-absorbable sutures require removal, leading to additional clinic visits, increased costs and redundancy compared with absorbable sutures. Additionally, there have been no studies addressing suture removal pain.
The two most common methods to close a surgical wound are absorbable and non-absorbable sutures. Some articles have been published on the subject comparing the two methods. Past RCTs comparing absorbable and non-absorbable sutures have primarily focused on assessing pillar pain and scar tenderness,12–14 while others also included steel sutures in their comparisons.15 The results of these studies are somewhat contradictory, and it remains unclear whether absorbable sutures perform differently from non-absorbable sutures. The limited sample sizes of just 33–64 patients in total increase the likelihood of coincidental findings and hinder the attainment of reliable results.
More recent studies have placed a greater emphasis on the aesthetic outcome of the scar.16 17 Nevertheless, even in these studies, the sample sizes were relatively small, with only 38 and 50 patients, and the follow-up periods were short. None of these studies found a significant difference between the groups compared. In 2010, a prospective cohort study on the aesthetic outcome after any elective day-case hand surgery was made. The study featured a more substantial sample size of 70 patients and found no statistically significant difference in the aesthetic outcome of the scar between absorbable and non-absorbable sutures.18 However, it is worth noting that this study included other surgical operations beyond carpal tunnel release, and the follow-up period was relatively short at only 6 weeks.
Contrary to traditional teaching, absorbable sutures may lead to fewer cases of dehiscence, infections and fewer clinical encounters related to wound-related concerns.19 In conclusion, there is not enough evidence for clinicians to make well-informed decisions regarding the choice of suture material. Thus, there is a clear and pressing need for a well-planned, well-executed and adequately powered RCT with reasonably long follow-up periods to compare non-absorbable and absorbable sutures.
Objectives
Our primary objective is to ascertain whether an absorbable suture is non-inferior to a non-absorbable suture concerning the aesthetic outcome of the scar 1 year after the operation. The secondary objective is to examine postoperative pain in the scar area, relief of symptoms, patient satisfaction, costs and safety.
Trial design
The trial is designed as a randomised controlled non-inferiority trial with two parallel trial groups with a 1:1 allocation ratio. A blinded outcome assessor is used and the triallists are blinded for the group assignments in data analysis phase.
Methods
The Standard Protocol Items: Recommendations for Interventional Trials statement was followed in this protocol.20
Trial setting
The trial will be conducted at Kuopio University Hospital in the Department of Orthopaedics, Traumatology and Hand Surgery which serves as a tertiary care unit in Eastern Finland.
Eligibility criteria
This trial aims to be practical and readily applicable to common treatment practices. Therefore, it is crucial to compile a trial population that closely mirrors the average patient undergoing median nerve release. We do not exclude patients based on gender, ethnicity or any specific medical condition.21 Nevertheless, some technical considerations restrict the trial population. Furthermore, a few factors have the potential to obscure the results or hinder the operation’s success. These are detailed in the exclusion criteria (table 1).
Eligibility criteria for the trial
Interventions
The patients participating in the trial will be randomly allocated to one of two equal groups and treated with either the absorbable or the non-absorbable suture. Regardless of the trial group, the operation will be conducted in the same manner. The operating surgeons will make a skin incision distally from the distal wrist crease and ulnar to the thenar crease. The appropriate tissues including the subcutaneous tissue, palmar fascia, flexor retinaculum and antebrachial fascia will then be divided. After the release of the median nerve is complete, the incision will be closed by the randomly determined type of transcutaneous suture, with simple single stitches 0.6 cm apart from each other (figure 1). The sutures used are a 5-0 Vicryl Rapide (Ethicon, Raritan, New Jersey, USA) absorbable suture and a 5-0 Dafilon (B Braun Melsungen, Melsungen, Germany) non-absorbable suture. The operating surgeons will be informed of appropriate wound closure. At the end, a light surgical dressing is applied to the hand, to be removed after a few days based on the surgeon’s evaluation. The patient has the option to either remove the dressing themselves or seek assistance from a healthcare professional, such as a nurse from basic or occupational healthcare. Patient are instructed to start immediate use of the operated hand, but heavier hand use is recommended to be avoided for 2 weeks. The patient is advised to schedule the removal of non-absorbable sutures at the basic or occupational healthcare centre after 2 weeks. For absorbable sutures, patients are encouraged to wipe the wound with a coarse towel after 2 weeks if the stitches have not naturally fallen out. If the absorbable sutures do not fall out even with this procedure, patients are instructed to seek assistance from a healthcare professional. After surgery, the nurse provides self-care instructions to the patient and reviews them (online supplemental appendix 1). Non-steroidal anti-inflammatory drugs or paracetamol is used for post-surgical topical pain management.
Supplemental material
Carpal tunnel release skin incision closed with (A) non-absorbable and (B) absorbable sutures.
Modifications
The treatment has been performed for decades. Therefore, all the initiated operations are likely to be completed without any modifications.
Adherence
Specific measures have been implemented to facilitate trial adherence. The trial flow and guidelines will be comprehensively explained to the participants in advance, and they will receive all the necessary information in written form. Preoperative surveys will be completed with the recruiter. Two weeks after the surgery, the participants will receive the first survey via email, which will include additional instructions for response. In cases of non-adherence, participants will be contacted directly. One year after the surgery, a face-to-face appointment will be scheduled during which all remaining surveys will be completed. Participants can contact the trial officials at any time. The appointment letters will be sent to the patients and those who miss their appointments will be offered rescheduled appointments. If a patient is unable to attend the follow-up visit in person, the necessary information can be collected over the phone. Patients can send a picture of the scar to the research nurse and the scar will be evaluated based on the picture.
Outcomes
Primary outcome measures
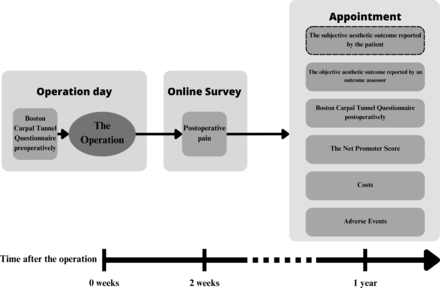
The primary outcome will be the mean difference between the two study arms in the aesthetic outcome of the scar, which will be assessed on a 10 cm Visual Analogue Scale (VAS) ranging from ‘the ugliest scar possible’ to ‘the most beautiful scar possible’. The evaluation will be performed by the patient 1 year after the operation.
The patient has been selected as the outcome assessor for the primary outcome measure, as the primary aim of the trial is to enhance patient satisfaction with carpal tunnel release surgery. The VAS was selected to avoid overemphasising scar assessment items that may not contribute to patient satisfaction. It is widely used in clinical practice and is straightforward for the patient to complete.22
Secondary outcome measures
With the secondary outcome measures, we will assess the scar with a blinded outcome assessor, postoperative pain, the subjective result of the treatment using the Boston Carpal Tunnel Questionnaire patient satisfaction by the Net Promoter Score (NPS), costs and safety (table 2).
Secondary outcome measures
Participant timeline and recruitment
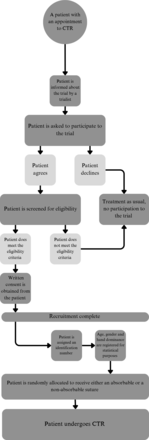
Recruited patients will be individuals attending the outpatient clinic of Orthopaedics, Traumatology and Hand Surgery at Kuopio University Hospital. Recruitment commenced on 21 September 2022 and is anticipated to continue until the end of 2025. The recruitment and screening process will occur before each patient’s operation, during which the recruiter will inform the patient about the trial. Eligible patients who provide consent may then enrol in the trial by completing the trial consent form. The recruiter will assist the patient in filling out the preoperative questionnaire. A postoperative questionnaire will be sent to the patient via email. One year postoperatively, patients will be scheduled for an appointment during which they will complete the remaining postoperative surveys with a trial nurse. An outcome assessor will assess scar aesthetics using a dedicated form (figure 2).
Participant recruitment flow chart. CTR, carpal tunnel release.
Sample size
Based on an assessment of clinical wound evaluation scales, a VAS cosmesis scale should be able to detect the minimal clinically important difference (MCID) of 15 points on a 100 mm VAS.23 The non-inferiority margin was set to 10 points considering the MCID and by using clinical judgement. Assuming a common SD of 20 points, a sample size of 50 patients per group is required to have 80% confidence that the lower limit of a one-sided 95% CI will be above the non-inferiority margin of −10 points.24 To account for a 15% attrition rate, the group size is increased to a final size of 58 patients. Thus, the total sample size is 116 patients.
Allocation
Participants will be randomly assigned to one of two experimental groups with a 1:1 ratio and stratified by hand dominance. Two randomised computer-generated stratification lists will be created before the recruitment phase and will not be accessed by those involved in recruitment or allocation. Allocation will be conducted by a specific nurse via phone, who is not otherwise associated with the enrolment process. While the nature of the intervention will eventually make the patient, recruiter and care providers aware of the trial group, the outcome assessor evaluating scar aesthetics at the 1-year follow-up will remain blinded to the allocation.
Blinding
Participants receiving non-absorbable sutures will be aware of the suture type due to the need for an additional appointment to remove them. Similarly, the surgeon and the other care providers will be aware of the suture type. It is therefore impossible to blind these groups to the intervention. Instead, a blinded outcome assessor will be used at the 1-year follow-up visit. They will not be in contact with the patient prior or after the evaluation. The allocation is not revealed to them by the nursing staff or the patient, and they do not have permission to access the patient’s medical reports and will thus be unaware of the allocation. Additionally, the triallists performing data analysis will be blinded to the group assignments.
Data collection methods
Data will be collected directly from the patient in the form of paper and online surveys. Participants retain the right to revoke their consent and withdraw from the trial at any time. In such cases, data collection will cease, but all data collected up to that point will be retained and used.
Primary outcome
The scar cosmesis-evaluating scale will be completed at the 1-year appointment with a nurse. The nurse will measure the VAS result and log the number (mm) into an online survey. For this trial, SurveyMonkey (Momentive, 1999, San Mateo, California, USA) will be used for the online surveys.
Secondary outcomes
Participants will complete the Boston Carpal Tunnel Questionnaire with the recruiting staff member preoperatively on a paper form (figure 3). The same process will happen at the postoperative 1-year appointment, with a nurse present.
Data collection timeline.
The participants will be sent an online survey via email present 2 weeks after the operation. In the survey, the participants are asked to evaluate pain around the scar during the past week on a VAS pain scale. The participants are explicitly instructed to include pain caused by potential suture removal in this assessment.
At the 1-year appointment, the outcome assessor will visit the consulting room to assess the aesthetic outcome of the scar on a VAS similar to the patient’s. The result will be measured by the nurse and logged online. Additionally, the participants will complete the NPS survey on a paper form. Costs will be assessed based on healthcare resources used, extracted from trial data and relevant registries. Any potential adverse events will be inquired about and recorded in the 1-year follow-up questionnaires.
Retention
The 1-year-long follow-up period is a risk for non-retention. After 2 weeks, participants will answer an online survey independently. To reduce non-retention, all other surveys will be answered in the presence of a triallist. The ability of participants to receive information and answer surveys online has been considered during enrolment. Absence from the 1-year appointment is a possible way to lose participants to follow-up. However, the recruiters will be instructed to emphasise the importance of the appointment before the patients consent to participate. The presence of a triallist at most of the data collecting points should diminish non-retention. A triallist and a trial nurse will actively monitor the progress of patients in the trial and non-adherent patients will be actively contacted by phone. At recruitment, patients will receive written instructions on how to contact the trial personnel in case of queries regarding the trial.
Data management
Surveys completed in the presence of staff members are in paper form. All the data from both SurveyMonkey and paper forms are transferred into R Statistical Software (V.4.3.1; R Core Team 2023) after the recruitment phase ensuring that all data are input at least twice.
All personal information about the enrolled participants will be stored in a locked cabinet at the trial site with access limited to trial personnel. All logs containing personal information to identify a participant are stored in a separate file. The written consent forms and all collected surveys are similarly stored in their respective files separately. The online survey database is protected by two-factor identification. Access to all stored information is limited to the authors.
Statistical methods
Aesthetic VAS will be used as the outcome variable, with group allocation as the independent variable, and age, gender and hand dominance as covariates as these may be prognostic for the main outcome. The primary analysis will involve age and gender-adjusted group differences, with the crude group difference reported based on the Welch t-test. Linear regression analysis will be used to estimate the treatment effect.
To minimise potential bias in interpreting the findings, the triallist, who will be blinded to the treatment allocation, performs data analysis. Blinded results (groups A and B) will be presented to the writing committee, where a collective consensus on the interpretation of the findings will be reached. Once a consensus is achieved, the groups will be unblinded.25
Data monitoring
No new or experimental treatments are being conducted, and both suture types under study are commonly used in open carpal tunnel release surgery. The associated risks can be considered minimal. Therefore, a formal data monitoring committee is not required. There will be no interim analysis to terminate the trial for similar reasons.
Harms
Adverse events will be monitored at the 1-year appointment. Patients are instructed to immediately report potential serious adverse events to the Department of Orthopaedics, Traumatology and Hand Surgery at Kuopio University Hospital. Adverse events include, for example, scar tearing requiring medical attention or a superficial infection necessitating oral antibiotic treatment. Serious adverse events include, for example, deep scar infection requiring hospital care, nerve, tendon or arterial injury, and complex regional pain syndrome. All adverse events are promptly treated with necessary measures, following standard treatment protocols, as both wound suture methods used have been proven to be safe.
Patient and public involvement
Patients were not actively involved in the trial.
Ethics and dissemination
Research ethics approval
The trial protocol and materials distributed to participants were approved by the Medical Research Ethics Committee of Wellbeing Services County of Northern Savo (5.1.2022). Any potential modifications to the research plan will also be submitted to the Research Ethics Committee following their guidelines. Additionally, any changes will be communicated to other potential trial participants if relevant.
Consent
Informed consent will be obtained from patients by a designated group of recruiters during the recruitment phase of the trial. The recruiting staff include members of the research team and a trial nurse, all of whom will undergo proper training and receive written instructions before the enrolment begins. The nature of the trial will be thoroughly explained to potential participants and written informed consent will be obtained by the recruiter.
Dissemination policy
The results of the trial will be published in a peer-reviewed journal, and all participating patients and healthcare workers will be informed about the article. Access to the article will be arranged if necessary. The pseudonymised data supporting the findings stated in the results article are available upon request from the corresponding author (AS) (online supplemental table 1).
Discussion
Carpal tunnel release surgery is a common and essential treatment due to the relatively high prevalence of CTS in the general population. Therefore, it is highly appropriate to optimise the procedure as far as possible. This trial focuses on absorbable and non-absorbable sutures, both widely used in open carpal tunnel release surgery.
Previous studies have investigated the relationship between suture material and scar cosmesis in carpal tunnel release surgery. However, these studies had limitations. For instance, a prospective cohort study by Dosani et al 17 using the Stonybrook Scar Evaluation Scale found no statistical significance between the two most used suture materials. Similarly, a prospective cohort study by Kundra et al 18 and an RCT by Theopold et al 16 did not yield different results. These past studies had relatively small sample sizes of 70 and 38, limiting their statistical power. Similarly, the follow-up times were 3 months and 6 weeks, respectively. Postoperatively, the scar undergoes changes and requires time to heal to its more stable form.26 Therefore, a longer follow-up period is necessary to account for individual differences in wound healing.
Aside from the method of wound closure, various factors can affect the patient’s overall experience. One such factor is the pain caused by the removal process of the non-absorbable suture, which must be considered when measuring postoperative pain as it can significantly impact patient satisfaction.
Due to the nature of the intervention being studied, it is not possible to blind the recruiting staff, the patients or the healthcare workers to the treatment. To address this limitation, a specifically assigned blinded outcome assessor is used to mitigate potential subjective biases that could skew the results.
This study aims to be practical and easily applicable to common treatment practices. The wound healing process is lengthy, and it may take up to a year for the resulting scar to reach its final form.26 This aspect is considered in the trial’s design. The trial’s strengths include evaluating results at 1 year postoperatively, allowing most patients’ scars to settle. Additionally, the trial features a relatively large sample size compared with previous studies on the subject, enhancing the likelihood of detecting clinically relevant results.
This trial seeks to address the limitations of past studies on this topic. A Cochrane literature review by Wade et al 11 found similar conclusions, highlighting the need for a high-quality RCT with sufficient evidence to assist healthcare professionals in making informed decisions regarding suture material choice. This trial aims to address this identified need.
Ethics statements
Patient consent for publication
Acknowledgments
We are deeply grateful for trial nurse Elina Jalava for her remarkable efforts towards the trial and the personnel at the outpatient clinic at the Kuopio University Hospital Department of Orthopaedics, Traumatology and Hand Surgery for making the trial possible.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @raisanen_mikko
Contributors AS—corresponding author, planning, writing and submitting the protocol. YN—planning, reviewing the protocol and steering the primary author. AR—planning and writing the statistical analysis plan. JS—reviewing the protocol and planning the data management and collection. MH—writing the protocol. NH—writing the protocol.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests AS, YN, AR, JS, MH and NH—none. MPR—hand and wrist course, Arthrex.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.