Article Text
Abstract
Introduction Surgical patients over 70 experience postoperative delirium (POD) complications in up to 50% of procedures. Sleep/circadian disruption has emerged as a potential risk factor for POD in epidemiological studies. This protocol presents a single-site, prospective observational study designed to examine the relationship between sleep/circadian regulation and POD and how this association could be moderated or mediated by Alzheimer’s disease (AD) pathology and genetic risk for AD.
Methods and analysis Study staff members will screen for eligible patients (age ≥70) seeking joint replacement or spinal surgery at Massachusetts General Hospital (MGH). At the inclusion visit, patients will be asked a series of questionnaires related to sleep and cognition, conduct a four-lead ECG recording and be fitted for an actigraphy watch to wear for 7 days before surgery. Blood samples will be collected preoperatively and postoperatively and will be used to gather information about AD variant genes (APOE-ε4) and AD-related pathology (total and phosphorylated tau). Confusion Assessment Method-Scale and Montreal Cognitive Assessment will be completed twice daily for 3 days after surgery. Seven-day actigraphy assessments and Patient-Reported Outcomes Measurement Information System questionnaires will be performed 1, 3 and 12 months after surgery. Relevant patient clinical data will be monitored and recorded throughout the study.
Ethics and dissemination This study is approved by the IRB at MGH, Boston, and it is registered with the US National Institutes of Health on ClinicalTrials.gov (NCT06052397). Plans for dissemination include conference presentations at a variety of scientific institutions. Results from this study are intended to be published in peer-reviewed journals. Relevant updates will be made available on ClinicalTrials.gov.
Trial registration number NCT06052397.
- Anaesthesia in neurology
- Dementia
- Delirium & cognitive disorders
- Sleep medicine
- GENETICS
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Objective study of sleep and circadian rhythms before and after surgery.
Quantifies novel markers of Alzheimer’s disease pathology.
Explores the role of APOE-ε4 in perioperative sleep and postoperative delirium.
Observational nature of study cannot demonstrate causality.
Study burdens for patients after surgery may increase missing data.
Introduction
Postoperative delirium (POD) is one of the most common complications following surgery for older adults, occurring in 5%–50% of cases.1 POD is a neurocognitive syndrome with acute attention, cognitive and awareness deficits that develops over a short period of time and has a fluctuating course. It is associated with cognitive dysfunction2 and Alzheimer’s disease (AD) and related dementias (ADRD) but can present in otherwise cognitively intact older adults. Millions of older Americans (≥70 years) require major surgery each year and are exposed to the risks of POD and its consequences.3 Furthermore, there are currently no treatments for POD, but it perpetuates a cascade of poor health outcomes costing approximately US$50 billion annually.4 Older age and baseline cognitive impairment (eg, pre-existing AD/ADRD) are major risk factors. Male gender, multiple comorbidities, sensory impairments, sleep deprivation and use of sedative medications are also potential risk factors.5–7 POD may also predispose to AD/ADRD, suggesting overlapping pathophysiology.3 8–10 Yet, minimal attention or cognitive follow-up exists for those who suffer from POD.11 Given that nearly 40% of POD is preventable with attention to multifactorial preoperative care,12 the search for modifiable risk factors and novel biomarkers in POD is of great public health importance.
At the same time, there is a silent epidemic of treatable chronic sleep problems and circadian disruption (ie, shifting of the body’s daily biological rhythm) affecting over 70 million older Americans. Such symptoms of these issues include insufficient sleep, irregular timing, unscheduled naps, insomnia or daytime sleepiness.13 Sleep/circadian disruption increases with age and cognitive impairment and is associated with frailty,14 pain and opioid use,15 preclinical AD pathology16 17 and the onset of AD/ADRD.18 POD is associated with disruption of melatonin secretion—the key circadian hormone in sleep homoeostasis,19 particularly, in older patients.20 Despite this, there is minimal consideration of sleep/circadian disruption in preoperative medicine. Pre-existing sleep/circadian disruptions are likely to be exacerbated during recovery after major surgery.21
Our recent work and that of others suggest that suboptimal sleep/circadian regulation is associated with POD risk22–26 and progression to dementia, independent of age, sex, education and cognition.18 These studies also uncovered that sleep/circadian measures correlated with cerebrospinal fluid amyloid/tau burden decades before dementia onset,16 17 and that plasma tau burden was associated with POD in two surgical cohorts.27 28 Finally, genetic biomarkers, namely the APOE-ε4 genotype, may moderate the relationship between sleep and AD risk,29 but its role in POD remains controversial.30 31 While epidemiological evidence points to sleep/circadian disruption as a shared modifiable risk factor for POD and dementia, direct clinical evidence is lacking.
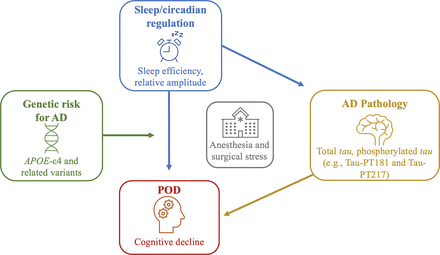
This leads us to our central hypothesis that sleep/circadian disruption promotes cognitive vulnerability after anaesthesia/surgery via increased AD pathology, and this relationship is exacerbated by genetic risk for AD via APOE-ε4 or a polygenic risk score (see figure 1 for the conceptual model). To test this hypothesis, we propose the SLEEP-POD study—a single-site, prospective, observational study of 150 older patients (≥70 years) undergoing elective, major orthopaedic surgery at the Massachusetts General Hospital (MGH). This study will assess preoperative sleep and circadian rest/activity rhythms using validated sleep questionnaires and objective week-long actigraphy measures. Cognitive assessments will be performed throughout the study. Blood samples will be retrieved to gather genotyping data and tau measurements.27 28 The primary outcome will be the presence of POD before discharge or within 3 days of surgery. Additional actigraphy assessments and evaluations for cognition, sleep and pain will occur periodically during follow-up visits at 1, 3 and 12 months after surgery.
SLEEP-POD conceptual model: sleep/circadian disruption effects on POD via plasma AD pathology burden and influence of genetic risk of AD. AD, Alzheimer’s disease; APOE-ε4: apolipoprotein ε4; POD, postoperative delirium.
Methods and analysis
Study design
This protocol presents a single-site, prospective, observational study to examine the relationship between sleep/circadian regulation and POD and how this association is moderated or mediated by tau and genetic risk for AD. Subjects meeting the eligibility criteria listed in table 1 will be recruited after obtaining written informed consent. Recruitment will take place at MGH. A trained study staff member will carry out the process of consenting patients. The study protocol and assessments to be performed are presented in table 2.
Inclusion and exclusion criteria with rationale
Study flow chart
Study registration
This study is approved by the Institutional Review Board (IRB) at MGH, Boston. This study is registered with the US National Institutes of Health on ClinicalTrials.gov (NCT06052397). The study is expected to be open for recruitment for 3 years.
Inclusion and exclusion criteria
We will include 150 older (≥70 years) elective orthopaedic surgical patients undergoing joint replacement or spine surgery at MGH with an expected postoperative recovery of greater than 24 hours. Exclusion criteria are as follows: known severe dementia (diagnosis or Mini-Mental State Examination (MMSE) score <18) or related treatment, alcohol/drug abuse within 2 years, inability to wear an actiwatch, need for urgent/emergent surgery or surgery in the prior month, more than 2-day intensive care unit (ICU) stay in the prior month, or insufficient vision, hearing, or English language for testing completion. A complete listing of trial exclusions and rationale is found in table 1.
Recruitment
Trained members of the study team will identify potential subjects presenting to MGH for orthopaedic surgery. Whenever possible, a study team member (clinical research coordinator, principal investigator (PI) or coinvestigator) will meet the patient during the orthopaedic surgery clinic visit before surgery. The study procedures will be explained in detail, and consent will be obtained from interested participants. Eligible patients whom we cannot connect with at their preoperative clinic visit will be contacted via telephone. For interested participants, consent will be obtained electronically, and directions for obtaining the actiwatch will be explained to them.
Sleep/circadian instruments (actigraphy, sleep diary and questionnaires)
In preoperative clinics, patients will complete sleep quality questionnaires (eg, Pittsburgh Sleep Quality Index) and be instructed to complete an electronic sleep diary while wearing a validated wristwatch (Actiwatch Spectrum Plus) for 7 days (table 2). We will apply algorithms and quality control best practices32 to objectively infer rest-activity rhythm measures from the accelerometer data. The watch will collect data that will be used to calculate estimations of sleep duration, regularity, stability and timing of activity rhythms. Patients will return the watch on the day of surgery and will be asked to wear it for an additional week at their 1, 3 and 12 months follow-up visits.
Sleep/circadian measures
The data collection methods described above inform how we will test our hypothesis. The exposures enlisted in this study can be broken down into coprimary exposures relating to sleep and circadian disruption and subsequent exploratory exposures associated with sleep/circadian disruption.
Sleep efficiency (SE) will test how sleep disruption materialises on testing our hypothesis. The data collected from the sleep diary and actigraphy watch will inform the ratio for SE (SE=total time asleep/time spent in bed).33 Secondary/exploratory exposures for sleep analysis include sleep duration (total sleep time), sleep latency (time taken to fall asleep), wake-after-sleep-onset (WASO), sleep fragmentation (kRA) and daytime sleepiness or napping.33–35
Relative amplitude (RA) will act as the exposure for identifying how circadian disruption influences the relationship tested in our hypothesis. The actigraphy-derived measures M10 (the most active 10-hour period per day) and L5 (the least active 5-hour period per day) will inform RA (M10-L5/M10+L5).18 Secondary/exploratory exposures for circadian analysis include phase (peak activity time in hours during a 24-hour period), MESOR (midline-estimating statistic of rhythm or rhythm-adjusted mean of a 24-hour period), interdaily stability (IS; stability of between-day rhythms) and intradaily variability (IV; within-day fragmentation of rhythms).36–38
DNA and plasma collection
Blood samples will be collected preoperatively and postoperatively via indwelling lines, and a nutrition assessment will be conducted before all blood collections to assess the nutritional impact on the collected blood samples. The samples will be used to gather information about AD variant genes, including APOE-ε4, and investigate associations with baseline and changes in plasma AD-related pathology (total and phosphorylated tau). Tau will be measured using a nanotechnology platform,27 28 which employs 20 000 nanoneedles to quantify phosphorylated tau at a very low abundance in blood samples. Phospho-specific antibodies identify tau (eg, Tau-PT217, Tau-PT181) and produce a colour shift that is recognised by digital software. The software uses a threshold for colour shift to determine if a binding event is positive (ie, Poisson statistics).
Clinical data collection
Clinical data, including age, sex, body mass index, comorbidities, length of hospital stay and surgical details (eg, surgery duration, anaesthesia type, blood loss, complications), will be recorded using passively collected data from the patient’s electronic medical record.
Study outcome measures
The primary outcome of this study is the presence of delirium up to day three or discharge after major orthopaedic surgery. Delirium will be assessed using the Confusion Assessment Method (CAM) or CAM-ICU, which will be scored by the study team members twice daily on postoperative days 1–3. The CAM considers performance on attention, orientation and memory tests from the Montreal Cognitive Assessment (MoCA) and responses to the Delirium Severity Index—a symptom severity questionnaire. Trained study staff will use these data to complete the long CAM, informing a diagnostic algorithm determining delirium status at the assessment time. The presence or absence of delirium during postoperative days 1–3 or before discharge will be recorded binarily.
Secondary outcomes of this study include the following:
Severity of delirium will be measured using the CAM-S up until postoperative day 3 or discharge. CAM-S ranges from 0 to 19 points, with larger points indicating more marked delirium features.
CAM will measure delirium-free days up until postoperative day 3 or discharge.
Postoperative cognitive status will be recorded via telephone 1, 3 and 12 months after surgery using the Telephonic MoCA.
Postoperative health-related quality of life will be measured using a series of questionnaires from the Patient-Reported Outcomes Measurement Information System that evaluate global health (Short Form (SF) V.1.1), physical function (SF 8b V.1.2), pain interference (SF 8a V.1.0), applied cognition abilities (SF 8a V.1.0) and sleep disturbance (SF 4A V.1.0) at 1 month, 3 months and 12 months after surgery.
Other outcome measures: Postoperative sleep and rest-activity rhythm data from accelerometer watches will be assessed as an exploratory outcome measure. In addition, patients will be evaluated during the baseline interview with continuous ECG recording to explore heart rate response and vascular biomarkers associated with delirium.39 40 A four-lead ECG with oximetry will be placed on the patients by trained study staff during the inclusion visit. We will explore pulse wave velocity and augmentation index (AI) to evaluate artery stiffness and peripheral resistance, given potential relationships to sleep health, ageing and pathogenesis of ADRDs that may overlap with delirium.41 42
Data analysis
We will construct logistic regression models to test the association between the coprimary exposures (SE and RA) and POD. If exposures are uncorrelated, we will apply Bonferroni correction (p=0.05/2). We will then explore the association between continuous exposures (ie, sleep duration, sleep latency, WASO, sleep fragmentation, daytime napping, circadian phase, MESOR, IS and IV) and POD and multiple linear regression models for continuous outcomes (ie, cognition, pain, mood43 and physical function; p<0.05). We will control for pre-existing sleep disruptions by implementing generalised mixed-effects models to explore how changes in sleep/circadian regulation at follow-up endpoints are related to cognitive changes and whether his relationship is affected by POD occurrence, as a covariate and/or interaction with preoperative sleep.
To test whether tau mediates the above relationship, we will bootstrap the indirect effect CIs, the optimal method from Fritz and Mackinnon’s work,44 which would provide >80% power to detect the medium-medium-zero effect sizes condition (α=0.39, β=0.39, τ′=0). We will also test whether tau burden interacts with sleep/circadian regulation associated with POD. We will introduce interaction terms between APOE-ε4 status and our two exposure variables into our models. The model fit and outlier assessment will be examined using formal fit criteria and model inspection. We will select covariates based on prior POD or AD studies and the strength of association/extent of confounding while avoiding model overfitting. We will assess a core model with age and sex and build an adjusted model with the optimal number of predictors after evaluating with the Akaike information criterion.
Data management and quality assurance
This study will employ a web-based portal for data quality and completeness that will be updated by study staff regularly. The portal will display the following variables for all patients: sex, race, adverse events, study-related data, etc. To ensure data are accurately and completely collected during the study, the PI will be responsible for ensuring the study protocol is being followed, the IRB has approved changes to the protocol, and all facilities are appropriate for the conduct of the study. Also, the PI will review subject records to determine whether the data collected is accurate, complete and current. A scientific review will occur annually and consist of a review of subject recruitment, staff training and quality control procedures. Monitoring progress reports will be submitted to regulatory and/or funding bodies (IRB, Alzheimer’s Association) as requested or required.
Patient and public involvement
None.
Limitations
Limitations of the study include its observational nature and, therefore, the inability to demonstrate causation. Treatment effects after elective joint surgery may affect the association between sleep/circadian rhythms and delirium. In addition, repeat follow-up 12 months after surgery may increase the likelihood of missing data. Despite these pitfalls, older orthopaedic patients have similar characteristics, which mitigates variability within this study. They also have a high comorbid burden of sleep disturbances, which can enrich the signal for POD and cognitive decline. To mitigate the likelihood of missing data, we will allow a robust window for assessment completion with repeated contact attempts during the follow-up period.
The study does not measure postoperative in-hospital sleep disruptions, which could also contribute to POD. While we do control for orthopaedic surgery type, duration and type of anaesthesia, other unmeasured hospitalisation-related factors may not be accounted for. Additionally, while we assess for sleep apnoea risk, we do not perform gold-standard diagnosis (eg, home sleep apnoea testing45), which has potential links to POD.46
Patient burden during the study could originate from discomfort while wearing the actiwatch and fatigue during cognitive assessments. When possible, blood collection will be performed while the patient is under general anaesthesia and extracted from an indwelling line to minimise patient discomfort. All other efforts will be made to ensure minimal patient discomfort and stress throughout the study.
We acknowledge that those with dementia are the most likely to experience POD and cognitive decline, and testing our hypothesis within this group is of great significance and would likely enrich the sample. However, to enhance the feasibility of the study at this time, we decided to exclude those with ‘severe’ dementia as categorised by the MMSE (<18) due to study burdens (eg, wearing the actigraphy watch, filling out the sleep diary and answering extensive questionnaires). We will use this initial study to design future cohorts that engage caregivers for this vulnerable population.
Ethics and dissemination
The PI of this study will be responsible for final decisions regarding changes to the protocol and will report such changes to the MGB IRB. Electronic patient information will be accessed only as necessary throughout the completion of the study. All data collected from the study will be accessible to the PI. Additional analyses will be performed to formulate predictive models for delirium using sleep/circadian patterns and genetic data. The main papers reporting information about this study will present primary and secondary outcomes, and manuscripts describing the mechanisms for delirium pathophysiology from substudies (ie, circadian/sleep disturbance, actigraphy, protein biomarkers) will also be published. Plans for dissemination include conference presentations at a variety of scientific institutions. Results from this study are intended to be published in peer-reviewed journals. Relevant updates will be made available on ClinicalTrials.gov.
In conclusion, this study will provide useful data and certain insights into the biomarkers for delirium through analysis of sleep/circadian disruption and AD pathology. We present a novel approach to understanding a modifiable sleep/circadian risk factor for POD and cognitive decline after surgery.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to acknowledge all members and patients involved in the conduct of this study.
References
Footnotes
ES and EG are joint first authors.
X @EFrancoGmd
Contributors ES, PL, AM, ESM, OA, ZX, KH and LG were responsible for conceptualising the trial design. ES, EG, AM and LG are responsible for recruitment, enrolment and data collection. ES, EG, SNB, PL, CG, AM, HD, SS, EF-G, RS, ESM, OA, ZX, KH and LG have critically revised the protocol and approved the final version.
Funding Alzheimer’s Association Clinician Scientist Fellowship (AACSF) grant (AACSF-23-1148490).
Competing interests ZX provided consulting services to Baxter Pharmaceutical company, Shanghai 9th and 10th Hospital, NanoMosaic, and Anesthesiology and Perioperative Science within the last 36 months. The authors have no other conflicts of interest to declare.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.