Article Text
Abstract
Introduction The new WHO Labour Care Guide (LCG), also regarded as the ‘next-generation partograph’, is a core component of 2018 WHO consolidated guidelines on intrapartum care for positive childbirth experience. The Ugandan Ministry of Health is in the process of adopting the new WHO LCG with no local context-specific data to inform this transition. We will explore potential barriers and facilitators to healthcare providers’ (HCPs) sustained engagement in labour monitoring in Mbarara city, Southwestern Uganda, and use the data to refine the new WHO LCG and develop a suitable implementation strategy to effectively integrate LCG into routine maternity care in Uganda. We shall then assess effectiveness, validity and other preliminary implementation outcomes of using the new LCG in detecting prolonged labour.
Methods and analysis The study will use a mixed-methods approach to identify key LCG user perspectives to refine and customise the WHO LCG among 120 HCPs and stakeholders involved in maternity care and labour monitoring within facilities in Southwestern Uganda. The refined prototype will be deployed and used to monitor labour in all 14 basic and comprehensive emergency obstetric and newborn care facilities in the study area. We will review labour outcomes of 520 patients monitored using the new LCG and compare these outcomes with a historical cohort of 520 patients monitored using the partograph. The main effectiveness outcome will be the proportion of women diagnosed with prolonged labour and/or obstructed labour.
Ethics and dissemination Ethical approval was obtained from the Mbarara University of Science and Technology Research Ethics Committee (MUST-2023-808) and Uganda National Council for Science and Technology (HS2864ES). We shall obtain written informed consent from each participant. The results of this study will be published in international peer-reviewed journals and presented to the Ugandan Ministry of Health as policy briefs and at selected national/international conferences.
Trial registration number NCT05979194.
- OBSTETRICS
- Fetal medicine
- Maternal medicine
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
We will use recommended methods for implementation science and the results will be reported using the Standards for Reporting Implementation Studies guidelines.
This study offers an opportunity to ascertain whether the new Labour Care Guide (LCG) tool is an effective decision-making tool to monitor labour, making the results potentially valuable to inform successful LCG rollout and long-term use.
We will employ a historical cohort to compare outcomes because it was not possible to conduct a cluster-controlled randomised trial, since some healthcare providers were already using the new WHO LCG in some facilities.
Introduction
Global maternal and neonatal death rates are unacceptably high1–3 and these deaths are due to complications of pregnancy and childbirth, mostly from preventable or treatable causes. Most of these deaths (94%) are within low-income and middle-income countries and could be prevented through timely interventions. In Uganda, the maternal and neonatal mortality remains high at 336 deaths per 100 000 live births and 27 deaths per 1000 live births, respectively.4 Up to three-quarters of maternal deaths in Uganda are linked to prolonged and obstructed labour; 90% of perinatal mortality following birth asphyxia is directly attributed to obstructed labour.5 6 A recent study in rural Malawi documented a 20% (645 of 3246) caesarean section rate, of whom 241 (37.4%) had an indication ‘prolonged first stage of labour’ on a partograph.7 Only 5% crossed the action line in the first stage of labour, and 12% had received oxytocin to augment labour. Adequate labour monitoring, with early identification of complications and their management, are vital processes towards improved quality of care and averting the unfavourable delivery outcomes including fetal, newborn and maternal deaths.8
Friedman’s partograph of 1954, also referred to as the partogram, is a labour progress/monitoring chart, graphically depicting the dilatation of the cervix or presenting the dilatation of the cervix against time in labour (figure 1). Alert and action lines were later added on this Friedman concept by Philpott and Castle in 1972 and aimed at identifying deviations from normal and guiding users towards early intervention. This partogram has been the gold standard for monitoring labour globally,9 and safe motherhood initiatives of the 1990s rolled it out as a universal tool for monitoring labour in an effort to prevent prolonged and obstructed labour. However, despite decades of healthcare provider (HCP) training, support and investment, rates of partograph utilisation, acceptability and appropriate use in making critical decisions during labour remain suboptimal in resource-limited settings, with correspondingly high incidences of obstructed labour, its associated complications, and ultimately sustained high and unacceptable stillbirth, maternal and neonatal mortalities.1 10 10–13 The partogram also offers subjective variations and assumes that all women progress at the same rate, which may affect intervention rate.14 A study of 527 parturients in Southwestern Uganda observed that 77.6% of clinical records actually contained a partograph, and an abysmal 4.2% had been completed to standard.13 In fact, according to Lugobe and colleagues, the partograph was most commonly used to record birth outcomes and not the actual monitoring of labour which it is intended.
The labour progress chart (partogram).
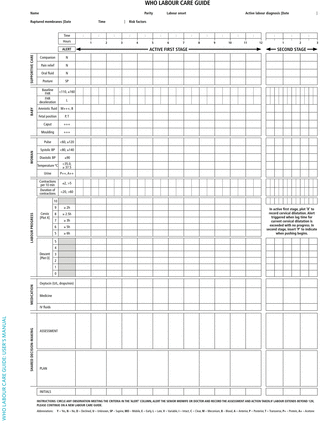
Use of effective evidence-based interventions during labour and childbirth, while avoiding ineffective or potentially harmful ones, could facilitate all women to achieve desired emotional, psychological and physical outcomes through regular assessments to identify any deviation from normality. With the persistent maternal/perinatal mortalities, the Ugandan Ministry of Health (MOH) has launched the new Essential Maternal and Newborn Clinical Care guidelines for Uganda in which the reproductive health experts recommended replacing the partograph with the new WHO Labour Care Guide (LCG).5 This recommendation was made with anticipation that the new tool was easy to use, promptly identifies deviations from normal through regular assessments, encourages self-efficacy, stimulates HCP interaction and shared decision-making, recognises participation of labour companions to promote women-centred care, but importantly the LCG has been developed for HCPs to identify deviations from normal through regular monitoring and assessment of women and their unborn babies. Based on pilot studies, this LCG has been suggested to facilitate HCPs to quickly identify any deviation from normality and thus improve labour monitoring as well as the well-being of women and their babies in comparison with the partograph.15 16 The LCG also has been suggested to encourage interaction between the healthcare team, women and labour companion or family members, while emphasising safety and providing the needed supportive care for the expectant women in our setting.17 In fact, according to Vogel and colleagues, this new WHO LCG monitoring tool is regarded as the ‘next-generation’ partograph incorporating recent effective intrapartum care guidelines.18 19 When used properly, the LCG is thought to accurately detect prolonged labour in time for HCPs to avoid unnecessary practices and interventions, and perform required interventions in time before progressing to obstructed labour and its sequelae, such as ruptured uterus, postpartum haemorrhage, sepsis, maternal and neonatal deaths. For example, while the partograph lacked clearly defined identifiers of prolonged/obstructed labour, the new LCG identifies grade 3 moulding and caput, all represented by +++, and further defines prolonged labour using cervical dilatation-specific time lags indicated in its ‘alert’ column of section 5 (figure 2) as per the new WHO consolidated guidelines on intrapartum care for positive childbirth.20 In fact, the new LCG has alerts for all observations that prompt the labour monitoring team to take action on each abnormal observation as soon as it happens, rather than waiting for the plot to reach action line as it is in the partograph.
The WHO Labour Care Guide.
Uganda’s MOH is in the process of rolling out the implementation of the LCG to all health facilities. However, there is no local context-specific data to inform this transition. Many new interventions have failed because of inattention to implementation needs early during their development. This study will employ evidence-based research frameworks to evaluate the effectiveness and process of LCG, through employing measurable implementation matrices (implementation, service, patient outcomes). This will help to identify potential opportunities and challenges, and inform and refine the implementation strategy and scale-up of this highly promising LCG. We will use best practices to develop a context-specific tool that is aimed at improving end-user (HCPs) acceptability, satisfaction, motivation, appropriateness, feasibility, fidelity, patient-centredness, penetration, user-centredness and effectiveness of this new tool in monitoring labour progress in a rural Southwestern Ugandan community setting where the impact of such an intervention is likely to be the greatest.
Methods and analysis
Study design
A mixed-methods approach will be used to collect data for this study. In-depth interviews and focus group discussions (FGDs) will be used to explore/identify key LCG user perspectives to refine and customise the WHO LCG for use in the Ugandan context. We will use the Consolidated Framework for Implementation Research21 (table 1), to refine LCG implementation strategies for future scale-up in Uganda or similar settings. The refined prototype will be deployed and used to monitor labour in all 14 basic and comprehensive emergency obstetric and newborn care facilities in Mbarara district and Mbarara city, Southwestern Uganda. We will review labour outcomes of 520 patients monitored using the new LCG and compare these outcomes with a historical cohort of 520 patients monitored using the partograph. The main effectiveness outcome will be the proportion of women diagnosed with prolonged labour and/or obstructed labour.
CFIR constructs that will guide data collection on intervention challenges, facilitators and potential strategies by HCPs and payers/managers
We plan to evaluate the LCG use in routine maternity care for preliminary implementation outcomes using Proctor’s implementation outcomes framework22 (table 2) across all public health centres and hospitals in Mbarara district and Mbarara city, Southwestern Uganda. Proctor’s framework provides a clear and simultaneous approach to differentiate and assess implementation outcomes from clinical and/or service outcomes, while providing a harmonised focus to guide developing plans for future large-scale implementation that accounts for individual, intervention, process, and inner and outer settings. Our selected outcomes of interest for this study will include feasibility, acceptability, satisfaction, motivation, appropriateness, fidelity, patient-centredness, penetration, user-centredness and effectiveness (outlined in table 2), which we hope will serve as indicators of implementation success or necessary preconditions for attaining desired service outcomes for users carrying out deliveries in rural, resource-limited settings. We hypothesise that the modified LCG will improve detection of prolonged labour and prevent obstructed labour and unnecessary interventions and their associated complications, ultimately reducing the maternal and neonatal deaths.
Application of Proctor’s framework to evaluate acceptability, appropriateness, user satisfaction, feasibility and fidelity
Study setting
The study will be carried out in all public health facilities offering basic and comprehensive emergency obstetric and newborn care in Mbarara district and Mbarara city. These include all 11 public health centre IIIs (HCIIIs) located in each subcounty, 2 health centre IVs (HCIVs) (Bwizibwera, Mbarara City Council) and Mbarara Regional Referral Hospital (MRRH). The district is served with a total of 253 HCPs who provide obstetric healthcare with a large concentration at MRRH. Each HCIV has 2–3 medical officers and 10 midwives on average,23 with HCIIIs having 3–5 midwives each.
The study will be conducted at the labour suite and postnatal wards of all the 11 HCIIIs, (six from Mbarara district including Bubaare, Bukiro, Kagongi, Kashare, Rubaya and Rubindi distributed in the six subcounties of Mbarara district and five HCIIIs of Biharwe, Kakoba, Nyakayojo, Nyamitanga and Kyarwabuganda distributed in the six division of Mbarara city plus two HCIVs (of Bwizibwera and Mbarara City) and MRRH. All mothers in labour are ideally monitored using a partogram and the fetal heart rate is measured manually per clinician judgement using the Pinard stethoscope. After a normal (uncomplicated) vaginal delivery, the mothers from these facilities with their babies are admitted to the postnatal wards for 24 hours, with daily ward rounds conducted by skilled birth attendants. Those who deliver by caesarean section remain admitted for 3 –5 days though mothers and babies with complications are admitted for more days.
Mbarara district is located approximately 270 km southwest of the capital, Kampala, with a population of about 250 000 people distributed through two recent administrative units of Mbarara city and Mbarara district. Uganda’s public health system is organised into seven tiers with national and regional referral hospitals, general district hospitals and four levels of community health centres. Staffing and available services vary across the four levels: HCIII offers basic emergency obstetric care (carry out prenatal care and conduct vaginal deliveries), whereas HCI and HCII serve as low-resource primary healthcare units. HCIVs and hospitals conduct normal vaginal and caesarean deliveries (offer comprehensive emergency obstetric care), and have ambulances and blood transfusion services.24 Private providers operate in parallel to the public health system to provide maternal healthcare. Basic and emergency obstetric care services are provided through four hospitals; MRRH and four privately owned: Divine Mercy Hospital, Ruharo Mission Hospital, Mbarara Community Hospital, Mayanja Memorial Hospital; and with two HCIVs of Bwizibwera and Mbarara city. Mbarara is served by 11 HCIIIs and over 40 privately owned health facilities that provide maternity services. The local economy of the districts is largely based on subsistence agriculture, with both food and water insecurity being common25; prenatal care attendance of ≥4 visits is still at 58%, and maternity services, including delivery, are largely provided free of charge.
Study population
We shall include adult HCPs actively involved in labour monitoring for at least 1 year in Mbarara district and Mbarara city with self-reported willingness to use the LCG. Officials from the reproductive health division of the MOH and health facility managers will be enrolled into the study. The principal investigator will provide a list of eligible HCPs and ministry officials to the research assistants, who will seek written informed consent (see online supplemental material) from each participant before enrolment. Each participant or surrogate will have a personal copy of the signed consent form.
Supplemental material
Eligibility criteria
Qualitative/stakeholder interviews
Adult HCPs exposed to the new LCG and MOH officials with self-reported willingness to use the LCG in monitoring labour and able to provide informed consent will be invited to participate in this study. The purpose of the study and study procedure shall be explained. A written informed consent form to participate and audio recording of the interview shall be obtained (see online supplemental material). Individuals unable or not willing to provide informed consent will not be eligible to participate in this study.
Quantitative data
Data from health facility records of mothers monitored using the partograph (historical cohort) and prospective data using the new LCG shall be abstracted to document study outcomes. A waiver of consent to review patient labour records shall be sought from the Mbarara University Research Ethics Committee.
Study procedures
Qualitative/stakeholder interviews
We will carry out 15 in-depth face-to-face interviews among adult HCPs exposed to the new LCG actively engaged in labour monitoring for a period of at least 1 year before introduction of the new LCG. Also, 15 officials and stakeholders from the reproductive health division of the MOH and health facility managers will be interviewed. Guided by the Consolidated Framework for Implementation Research (CFIR),21 we will identify unique needs, challenges, facilitators and patterns of potential and sustained uptake of the new LCG to monitor labour in Southwestern Uganda. We will also collect data on suitable implementation strategies to inform future integration of the new LCG into routine maternity care. Interviews are anticipated to last 60–90 min. Written informed consent will be obtained at the outset of each interview session (see online supplemental material). All qualitative interview sessions will be audio taped/recorded with the participant’s permission. Two research assistants will be present at each interview, with one moderating the interview as a second one keeps time and takes interview notes. Data will be collected until saturation point is achieved.
LCG prototype development and pilot testing
We will then use this qualitative HCP perspectives to characterise and refine or modify the new WHO LCG to develop the final prototype for evaluation. We will iteratively test the LCG prototype among three sets of 10 HCP users, the first of which would be the HCPs previously interviewed. The second and third sets will be (1) two FGDs and (2) two expert panels that will include individuals with expertise in obstetric care and labour monitoring locally and nationally. The aim is to refine and customise the tool for easy uptake and sustained utilisation within a known intervention development framework.26–28 Upon completion of each prototype, we will interview HCPs to obtain feedback on the ease of use, complexity, content, tool’s ability to engage, motivate, prepare, request or get support/attention as needed, cues to action/alerts/prompts, social support, and guidance on what to do at each stage for optimal and timely response. The local and MOH experts will be engaged to define and refine relevant components of the LCG. The final LCG prototype will be ready for evaluation in routine care on a bigger scale (Mbarara district and Mbarara city health facilities). We will also customise the existing training manuals developed by the WHO to suite within the local Ugandan context using this feedback. This is aimed at improving the skill, ease of use and appropriate utilisation of the developed LCG to maximise impact. Training on LCG use will generally aim at behavioural change communication; reference information and footnotes will be integrated within the LCG prints to facilitate exposure, awareness, accurate delivery, usability, comprehension and decision-making.26
Final LCG prototype evaluation
Once the refined prototype is completed, we will evaluate the LCG use by enrolling HCPs conducting deliveries from all basic and comprehensive emergency obstetric and newborn care facilities of Mbarara district and Mbarara city. We will use Proctor’s implementation outcome framework (see detailed methods outlined in table 2) to evaluate implementation outcomes of using the new LCG in routine maternity care that include acceptability, satisfaction, motivation, appropriateness, feasibility, fidelity, patient-centredness, penetration, user-centredness and effectiveness among HCPs actively involved in deliveries across Mbarara district and Mbarara city. An additional data abstraction tool to obtain outcome data from women monitored using the partograph (historical cohort) and prospective data for those that will be monitored using the new LCG will be designed and used in the document review to collect data from patient records. We will design a database and enter all partograph and LCG abstracted data. We will document and assess delivery outcomes such as diagnosis of prolonged labour, augmentation of labour, duration of active phase of labour, duration of second stage of labour, presence of labour companion, caesarean sections, episiotomies, third or fourth-degree perineal laceration, initiation of breast feeding, completion of partograph/LCG and other relevant maternal–fetal outcome events as described in the Data collection and analysis section. No identifying sociodemographic information about women will be collected, and the assessment of LCG use is primarily directed towards the HCPs for this work. A waiver to consent and use women’s records for the purpose of chart abstraction will be sought from the Mbarara University Research Ethics Committee.
Quantitative data
We will summarise clinical and demographic data obtained from document review of the patients monitored using the new modified LCG and those from a historical cohort of patients monitored using the partograph. The proportion of women diagnosed with prolonged labour and/or obstructed labour in the two groups (plus other secondary outcomes) will be used to determine preliminary implementation outcomes in all public health facilities in Mbarara district and Mbarara city. We will then assess the diagnostic validity of the new LCG versus the partogram as the presumed standard of care. To describe the performance of the LCG compared with the partogram, we will consider prolonged labour as a dichotomous outcome to assess the specificity and sensitivity of the LCG, and fit a receiver operating curve at different time points from 4 cm cervical dilatation (for partograph) and 5 cm cervical dilatation (for the new LCG) until vaginal delivery or decision to perform caesarean section.
Assessing the diagnostic accuracy of LCG in detecting obstructed labour
Finally, we will assess the diagnostic accuracy and predictability of the new LCG compared with the partogram in effectively detecting prolonged labour and reduce rates of obstructed labour and its associated complications among women delivering in Mbarara district and Mbarara city. We will use the effectiveness data to assess the specificity, sensitivity, and negative and positive predictive values of the new LCG versus partogram as the presumed standard of care. Because the key identifiers of obstructed labour were not clearly defined and indicated on the previous partogram, namely grade 3 moulding and caput, we will use prolonged labour as a quantifiable comparative measure. Prolonged labour will be defined using a partogram as labour progress that crossed the action line (figure 1) and cervical dilatation-specific time lags indicated in the ‘alert’ column when using the LCG (figure 2). Whereas prolonged labour was defined at the end as labour crossing the action line on the partograph with no ongoing prompts in between, the new LCG recommends ongoing practical observable ‘time lag’ at each specific centimetre of cervical dilatation as specified in the ‘alert’ column of section 5 which empowers HCP to make decisions before obstructed labour develops, indicated by grade 3 moulding/caput in section 3 of the new LCG.
Implementation strategy
First, we shall use CFIR-guided stakeholder qualitative research strategy to characterise/refine the new WHO LCG and introduce it as a decision-making tool for monitoring labour in Uganda. This is good timing since the Ugandan MOH has already recommended replacement of partograph with the new WHO LCG.5 Already developed and available WHO LCG training/users’ manuals, Ugandan MOH technical support supervision and mentorship guidelines will be used. HCPs and labour monitoring resources previously available for monitoring labour using the partograph shall be used. LCG-tailored training and on-site mentorship/coaching to improve HCPs’ responsiveness to LCG use, self-efficacy and ability to effectively and continuously use LCGs while ensuring intervention fidelity (extent to which intervention is delivered as intended) shall be provided to all HCPs at all study sites.
Outcomes
Primary outcome
The main primary effectiveness outcome will be the proportion of women diagnosed with prolonged labour and/or obstructed labour, defined as labour crossing the action line on the partograph and labour lasting more than a specified centimetre cervical dilatation ‘time lag’ in the alert column of section 5 of the LCG.
Secondary outcomes
Secondary outcomes are the proportion of women receiving obstetric interventions such as caesarean sections, episiotomies, third or fourth-degree perineal laceration, augmentation; rate of completeness of labour monitoring tool; having a fresh stillbirth; duration of first and second stages of labour; 5-minute APGAR score, need for resuscitation, blood transfusion, mode of delivery; initiation of breast feeding; obstetric complications diagnosed and /or managed during labour, childbirth or immediate post partum; ruptured uterus; postpartum haemorrhage; maternal/newborn sepsis; and maternal, fetal and newborn deaths.
Sample size and power calculations
Qualitative
This study will enrol all HCPs actively engaged in delivering women across public facilities offering basic and comprehensive obstetric care across Mbarara city and Mbarara district, health facility managers and officials from the reproductive health division of the MOH. Qualitative data in form of in-depth face-to-face interviews, FGDs, expert panels and exit interviews will be collected until saturation point is achieved.
Quantitative
Eight per cent of all maternal deaths in Uganda are linked to obstructed labour. Prolonged labour remains a major pathway to common labour complications such as obstructed labour, postpartum haemorrhage (including ruptured uterus), puerperal sepsis and obstetric fistula, among others.29 The actual morbidity/mortality percentage attributable to prolonged labour alone remains unknown. It is hypothesised that LCG will be able to detect/diagnose twice as much prolonged labour as the old tool (partograph). The primary effectiveness outcome will be the proportion of women with prolonged labour. Prolonged labour for our study will be defined as (1) labour crossing the action line on the partograph and (2) labour lasting more than a specified centimetre cervical dilatation ‘time lag’ in the alert column of section 5 of the LCG. We will use sample size calculation formulae for independent cohort studies. This function gives the minimum number of case subjects required to detect a true relative risk with power (power) and two-sided type I error probability (alpha). This sample size is also given as a continuity-corrected value intended for use with corrected Χ2 and Fisher’s exact tests.30–33 To test the primary effectiveness hypothesis, allowing for a two-sided type I error of 5%, 90% power and assuming a 10% attrition, it will require 520 participants to detect at least a 10% difference in prolonged labour when using an LCG (18%) than using the partograph (assumed 8% incidence). Data analysis will be conducted using STATA V.17 (Statacorp, College Station, Texas, USA). We will ensure completed data are collected through an inclusion checklist and timely cleaning to maintain the study’s power to detect a difference.
Data collection and analysis
Stakeholder interviews to inform iterative development of the LCG tool will start on 1 August 2023. A final prototype of the LCG will be deployed within the first 6 months of the study, then deployed in all study sites. Effectiveness, primary and other secondary outcomes data collection (online supplemental material) is expected to end on 30 June 2025. The demographic and clinical data will be collected from maternity records of women who have delivered within 1 year before and a year after implementation of the LCG including patient demographics (eg, age, gravidity, parity, gestational age), prenatal, antepartum high-risk morbidities, non-communicable diseases and HCP demographics: age, education, experience, self-efficacy. All data will be entered into REDCap and checked for completeness and quality by the principal investigator and any problems that arise will be resolved immediately.
Supplemental material
Charts of women whose labour was monitored using a partograph 1 year before introduction of the new WHO LCG will constitute a partograph-historical cohort (control arm), while those monitored using the new LCG will form the prospective LCG (intervention arm) of the study. We will first summarise health-related and sociodemographic data between arms. For our primary effectiveness outcomes, we will fit a multivariable logistic regression model, with study arm as the predictor of interest, and age, high-risk pregnancy and health facility at enrolment as a priori additional variables in the model, due to their strong association with the selected outcome.34–36 Although not designed to detect a difference, we will also explore additional secondary outcomes, as listed above.
We will summarise other implementation outcomes for the new LCG users (HCPs) using descriptive statistics. Success in the implementation survey data will be identified qualitatively and by the top tertile of relevant acceptability, feasibility, satisfaction and appropriateness scales. We will also describe the ranked implementation strategies selected by LCG users and key MOH stakeholders observed during the initial stakeholder and feedback interviews.
Lastly, we will calculate the positive and negative predictive values of prolonged labour, and summarise these in relation to the calculated prevalence, as well as the documented prevalence in Uganda. Data analysis will be conducted using STATA V.17. Findings will be presented as descriptive statistics, scatter plots and graphs; statistical significance will be considered at p≤0.05.
Qualitative analysis
All transcripts from the stakeholder and feedback interviews will be transcribed verbatim in English by two research assistants. The aim of this analysis will be to inductively construct categories describing multilevel factors and strategies that might influence LCG use and implementation. Qualitative analysis will be inductive, and a codebook will be developed through conventional content analysis.37 To ensure accuracy, transcripts will be coded to calculate an intercoder reliability kappa statistic using the NVivo software V.12 (Melbourne, Australia). We will begin the category construction process with repeated review of transcripts to identify relevant content. Identified content will serve as the basis for developing a coding scheme. Coded data will be iteratively reviewed and sorted to suggest categories under the general domains using CFIR framework. The categories developed from coded data will consist of descriptive labels, elaborating text to define and specify each category’s meaning, and illustrative quotes taken from the qualitative data. Demographic data will be used to describe the sample.
Patient and public involvement
There is no patient or public involvement in the study. This study will explore HCPs’ perspectives on the new WHO LCG and use iterative design method to develop the new tool. A community of HCPs monitoring labour at the study sites and MOH officials exposed to the new tool will be involved in its iterative development and subsequently in its use. No patients shall be recruited in the study. Results from this study will be disseminated to the HCPs and health officials to guide wide-scale use in Uganda and beyond.
Ethics and dissemination
Ethical approval was obtained from the Mbarara University of Science and Technology Research Ethics Committee (MUST-2023-808) and Uganda National Council for Science and Technology (HS2864ES). Study site administrative permission was from MRRH, Mbarara District Health Officer, Mbarara City Health Officer and the director clinical services at the MOH. We will obtain written informed consent from all study participants before enrolment in the study. The study was registered at ClinicalTrials.gov (NCT05979194) on 4 August 2023. The research outcomes from this study will be published in international peer-reviewed journals and presented to the Ugandan MOH as policy briefs and at selected national/international conferences.
Discussion
This study offers an opportunity to ascertain whether the new LCG tool is an effective decision-making tool to monitor labour among obstetric care providers in publicly funded facilities in low-income settings such as Uganda. The WHO LCG has been referred to as a ‘next-generation’ partograph for HCPs in adequately monitoring the well-being of women and babies during labour and childbirth, timely identifying any deviation from normal and facilitating HCP interaction, and stimulating shared decision-making for HCPs, labouring women and their companions/family thus facilitating timely management, quality of women-centred care and birthing experience. The reference thresholds for abnormal labour observations (for each monitored parameter) provided by the new LCG are meant to trigger specific actions, and thus targets to minimise overdiagnosis and underdiagnosis of abnormal labour events and the unnecessary use of interventions such as caesarean sections and augmentation.
The strengths of this study include the utilisation of recommended or appropriate methodology for developing implementation science projects and health research interventions and the results will be reported using the Standards for Reporting Implementation Studies checklist. The Ugandan MOH is in the process of adopting the new WHO LCG with no local context-specific data to inform this adoption and transition for use in routine care. This study therefore seeks to use best practices to support intervention adoption, uptake, implementation, integration and scale-up. No rigorous adoption and evaluation of the effectiveness and implementation of the new WHO LCG intervention have been conducted in Uganda or similar settings to inform stakeholders on successful rollout of the intervention on a large scale, and long term, making the results of this study valuable. This study will be conducted at a time when the Ugandan MOH will be recalling the use of a partogram, with some facilities already using the new WHO LCG, and so a better controlled cluster-randomised trial was not be possible, hence our choice to use a historical or retrospective cohort of partograph data to compare outcomes.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @#Mugyenyi76, @Byamugishajk10, @WTumuhimbise
Contributors MRG conceptualised the study as a PhD scholar, wrote the research protocol and wrote the first draft under the supervision of JB, EA, WT and FTY through all these processes. All authors reviewed the manuscript and approved it for submission.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.