Article Text
Abstract
Objectives Potentially harmful non-steroidal anti-inflammatory drugs (NSAIDs) utilisation persists at undesirable rates worldwide. The purpose of this paper is to review the literature on interventions to de-implement potentially harmful NSAIDs in healthcare settings and to suggest directions for future research.
Design Scoping review.
Data sources PubMed, CINAHL, Embase, Cochrane Central and Google Scholar (1 January 2000 to 31 May 2022).
Study selection Studies reporting on the effectiveness of interventions to systematically reduce potentially harmful NSAID utilisation in healthcare settings.
Data extraction Using Covidence systematic review software, we extracted study and intervention characteristics, including the effectiveness of interventions in reducing NSAID utilisation.
Results From 7818 articles initially identified, 68 were included in the review. Most studies took place in European countries (45.6%) or the USA (35.3%), with randomised controlled trial as the most common design (55.9%). Interventions were largely clinician-facing (76.2%) and delivered in primary care (60.2%) but were rarely (14.9%) guided by an implementation model, framework or theory. Academic detailing, clinical decision support or electronic medical record interventions, performance reports and pharmacist review were frequent approaches employed. NSAID use was most commonly classified as potentially harmful based on patients’ age (55.8%), history of gastrointestinal disorders (47.1%), or history of kidney disease (38.2%). Only 7.4% of interventions focused on over-the-counter (OTC) NSAIDs in addition to prescription. The majority of studies (76.2%) reported a reduction in the utilisation of potentially harmful NSAIDs. Few studies (5.9%) evaluated pain or quality of life following NSAIDs discontinuation.
Conclusion Many varied interventions to de-implement potentially harmful NSAIDs have been applied in healthcare settings worldwide. Based on these findings and identified knowledge gaps, further efforts to comprehensively evaluate the effectiveness of interventions and the combination of intervention characteristics associated with effective de-implementation are needed. In addition, future work should be guided by de-implementation theory, focus on OTC NSAIDs and incorporate patient-focused strategies and outcomes, including the evaluation of unintended consequences of the intervention.
- Primary Care
- Quality in health care
- Health & safety
- GERIATRIC MEDICINE
- Cardiovascular Disease
- Chronic Pain
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Primary Care
- Quality in health care
- Health & safety
- GERIATRIC MEDICINE
- Cardiovascular Disease
- Chronic Pain
STRENGTHS AND LIMITATIONS OF THIS STUDY
This scoping review identified 68 studies published during the 20+ year period during which all currently available NSAID classes were on the market in many countries.
Multiple characteristics of interventions and author-reported effectiveness are reported for each of the identified studies.
Interventions focused on only NSAIDs versus NSAIDs as one of multiple medications were included, but the literature search used to identify the latter was limited to published systematic and scoping reviews.
As a scoping review, this study did not systematically assess the quality of included studies.
Background
Non-steroidal anti-inflammatory drugs (NSAIDs) reduce pain and inflammation through inhibition of cyclooxygenase (COX-1 and -2) enzymes, thereby limiting the production of inflammatory prostaglandins.1 Representing 5%−10% of global medication utilisation, NSAIDs are commonly used to treat arthritis and musculoskeletal pain, injuries, headache and other sources of acute and chronic pain.2 There are six classes of NSAIDs: salicylates, propionic acid derivatives, acetic acid derivatives, enolic acid derivatives, anthranilic acid derivatives and selective COX-2 inhibitors.3 NSAIDs are available in prescription and over-the-counter (OTC) strengths, a variety of different formulations, and oral (most common), intravenous, injectable and topical forms. The use of NSAIDs has risen globally throughout the last 20 years,4–8 in part due to increasing rates of chronic and persistent pain and an increasing ageing population.
In addition to anti-inflammatory and analgesic properties, NSAIDs have numerous other physiologic effects, which differ by NSAIDs class. For example, NSAIDs can reduce the integrity of the gastrointestinal mucosal barrier and limit submucosal blood flow, increasing the risk of ulceration, haemorrhage or perforation, particularly among vulnerable individuals; COX-2 selective NSAIDs are associated with lower gastrointestinal risk.1 9 Taking NSAIDs can reduce renal blood flow, alter the fluid-electrolyte balance and increase the risk of acute kidney injury.10 Risk for and worsening of hypertension, heart failure and other cardiovascular issues have also been associated with regular NSAIDs use.10–13 In 2015, the US Food and Drug Administration updated the black box warning on OTC NSAIDS to include, ‘NSAIDs can increase the risk of heart attack or stroke in patients with or without heart disease or risk factors for heart disease…The risk of heart attack or stroke can occur as early as the first weeks of using an NSAID…There is an increased risk of heart failure with NSAID use’.14 Medical societies and professional organisations around the world have established recommendations for limiting or avoiding NSAIDs in certain high-risk populations (online supplemental file 1).
Supplemental material
Despite numerous long-standing recommendations, potentially harmful NSAIDs prescribing and OTC use persists globally.15–18 As an example, multiple studies show that up to 30% of patients with chronic kidney disease are prescribed long-term NSAIDs.19 20 This high-risk use has resulted in a substantial number of adverse events; NSAIDs are a leading cause of drug-related hospitalisations and mortality.21–23 The drivers of potentially harmful NSAIDs prescribing and use are complex and multilevel.24–26 Clinicians’ unfamiliarity with professional recommendations, clinical inertia, limited alternative options for pain management, lack of patient knowledge or understanding, and broad availability of OTC NSAIDs are just some of the factors involved. The evolving regulatory landscape also complicates NSAIDs practice patterns and decision-making (a timeline of major NSAIDs-related regulatory events and other key historical timepoints in the USA, as an example, is shown in table 1).27 28
Timeline of major regulatory events and other key historical timepoints of US NSAID history
Further efforts are needed to reduce the potential harm associated with prescription and OTC NSAIDs and promote safer pain management for high-risk patients. The purpose of this paper is to provide an overview of published interventions to de-implement potentially harmful NSAIDs in healthcare settings, identify knowledge gaps and suggest opportunities for subsequent interventions and future research related to NSAIDs de-implementation.
Methods
We performed a scoping review of the scientific and grey literature reporting on interventions to de-implement NSAIDs in healthcare settings. Our review was guided by the Preferred Reporting System for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Reviews.29 As a scoping review, this review is not eligible for PROSPERO registration, but the protocol was posted at https://osf.io/ywe62/ in January 2022.
Eligibility criteria
Eligible studies were published in English between 1 January 2000 and 31 May 2022, employed any study design and evaluated interventions administered with the goal of de-implementing potentially harmful NSAIDs in a healthcare setting. We selected 2000 as the earliest eligibility year since it was the first full year in which selective COX-2 inhibitors were available for use in several countries, including the USA and UK. Thus, all six NSAIDs classes were available throughout the study period.
‘Healthcare settings’ included any outpatient or inpatient healthcare environments within any medical specialty.
‘NSAIDs’ included prescription or OTC oral or topical NSAIDs. NSAIDs that are not approved for current use were included if they had been approved at any point during the study period. For example, although rofecoxib and valdecoxib were removed from the US market in 2005,30 they were included in the literature search. Aspirin taken for cardiovascular disease (CVD) prophylaxis (<100 mg) was not included since the recommended dose is lower than that commonly used for analgesic purposes. If the purpose of the intervention was to study an inappropriate prophylactic use of aspirin, an exception was made to include that study. To be included, studies must have reported NSAID prescribing or use rates before and after the intervention, at minimum.
‘Potentially harmful’ NSAIDs included those that were prescribed or taken in a manner inconsistent with professional recommendations or otherwise recognised as high-risk by the study authors.
‘Interventions’ were defined as ‘any activity or set of activities aimed at modifying a process, course of action or sequence of events in order to change one or several of their characteristics such as performance of expected outcome’, as described by the World Health Organization.31 Interventions were actively delivered to healthcare clinicians, healthcare teams or directly to patients. All interventions included in the study involved de-implementation of NSAIDs. Passive interventions such as policy changes were not included.
‘De-implementation’ was defined as the systematic reduction or elimination of potentially harmful NSAID prescribing or use, or the modification of some aspect of NSAID prescribing or use to improve safety and/or reduce risk of harm (eg, taking proton pump inhibitors in combination with NSAIDs).
‘Patient populations’ were limited to adults > 18 years of age. Patients with or without specific medical conditions and of any health status were included.
Search strategy
With the guidance of a professional librarian, we searched PubMed, CINAHL, Embase, Cochrane Central, Google Scholar and Google for [intervention OR program OR related MESH terms] + [de-implement OR deprescribe OR reduce OR related MESH term] + [nonsteroidal anti-inflammatory drug OR NSAID OR related MESH term] in Spring 2022 (full search strategy appears in online supplemental file 2). Studies were limited to articles or abstracts published between 1 January 2000 and 31 May 2022. Only studies written in or translated into English were included.
Studies identified in the search were uploaded as abstracts to Covidence (Melbourne, Australia), an online systematic review management platform. Duplicate studies were auto-identified by Covidence and deleted. Two members of the research team (MR and EO) independently screened all abstracts for inclusion in the review. Discrepancies were resolved by conference with a third team member (JE). Studies that passed the screening stage were moved to full-text review. Three members of the research team (MR, EO and ES) independently reviewed all full-text studies for alignment with eligibility criteria and reviewed reference lists for additional studies. Discrepancies were resolved via conference among the three reviewers.
Since the reference list review identified some studies that focused on NSAIDs as one of multiple medications addressed in de-implementation or deprescribing interventions that were not captured in our initial search, we performed a second PubMed, CINAHL, Embase, Cochrane Central and Google Scholar search for systematic and scoping review articles related to medication de-implementation, deprescribing or polypharmacy interventions published between 1 January 2000 and 31 May 2022. Abstracts identified in the search were reviewed by the lead author (MR) to eliminate reviews that did not meet inclusion criteria. Each review article was independently searched by two team members (EO and JT) for studies that included NSAIDs and met all other inclusion criteria. Discrepancies were resolved via conference among the two reviewers.
Data extraction
Studies that passed full-text review were moved to the charting/data extraction phase. Using the Covidence extraction framework, data were independently extracted by two team members (MA and ES). Two additional team members (MR and EO) downloaded the extracted data table from Covidence, independently checked a 25% data sample for accuracy and resolved discrepancies by consensus.
The following data were extracted for each study: publication year, country, study design, intervention setting, type of intervention (de-implementation approach), participants (eg, physicians, pharmacists and patients), NSAIDs involved in intervention (prescription and/or OTC; classes and/or specific medications), focus patient population and guiding model/framework/theory. Intervention types were categorised as academic detailing/clinician education, clinician financial incentives, electronic health record (EHR)/clinical decision support, patient counselling, patient education, performance feedback (a.k.a., audit and feedback), pharmacist medication review, practice facilitation or coaching or other as informed by the work of Cliff et al 32 and Colla et al 33 and the authors’ knowledge of the literature. During the analysis, we combined patient counselling and patient education since these categories were defined differently across studies, and because of substantial overlap in categories.
We documented the general effectiveness of the intervention in de-implementing NSAIDs (yes, no or no change) and any patient-focused outcomes evaluated in relation to the intervention. An intervention was scored as ‘yes’ for effectiveness if any significant (p <0.05) improvement in the utilisation or prescribing of any NSAID was reported. For multiple-drug interventions, we focused only on NSAIDs results.
Extracted data were integrated and synthesised into tables and figures based on data extraction elements listed above. The research team collectively appraised results to summarise the identified interventions and identify gaps in the literature.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
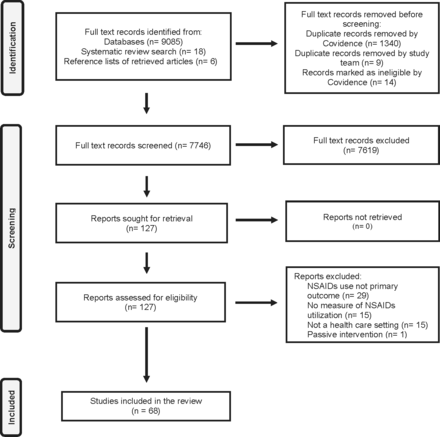
The original search identified 7720 studies from which 60 were included in the final review. The secondary systematic and scoping review search identified 98 articles from which eight additional papers were included in the final review. Figure 1 details the flow of articles through the identification and screening stages and online supplemental file 3 shows all articles included in the final review (n = 68).
PRISMA Flow Diagram.
Characteristics of studies
A total of 27 (39.7%) studies were published between 2000 and 2010, with the remaining published between 2011 and 31 May 2022. The majority of studies took place in a European country (45.6%) or the USA (35.3%) (online supplemental file 3). A variety of study designs were represented, with randomised controlled trial being the most common (55.9%) and prospective, interventional trials also frequently used (23.5%).
Characteristics of interventions
A minority of interventions (14.7%) were guided or informed by a specified conceptual theory, model or framework. Most interventions were delivered to clinicians (ie, clinician-facing) (76.5%) (online supplemental file 3a), although some were patient-facing (8.8%) (online supplemental file 3b) and some were both clinician and patient-facing (10.3%) (online supplemental file 3c). Of the clinician-facing and both clinician and patient-facing interventions, primary care or general practice physicians were the most frequent focus (72.6%), with pharmacists, nurses and physicians in sub-specialty settings the focus of the remaining interventions. Both single-component (54.4%) and multi-component (45.6%) interventions were employed. The most common intervention approach, represented in more than half of the studies, was academic detailing and/or clinician education (figure 2). Interventions focused on the EHR and/or clinical decision support were common among single-component interventions, while clinician performance reports or audit/feedback and medication review by a pharmacist were common among multi-component interventions (figure 2).
Single- and Multiple-Component Intervention Approaches to De-implement Potentially Harmful NSAIDS in Healthcare Settings. CDS, clinical decision support; EHR, electronic health record; NSAID, non-steroidal ant-inflammatory drug.
Some interventions focused solely on NSAIDs, while 26 (38.1%) focused on the de-implementation of other medications as well. For example, the EQUiPPED trial34 aimed to reduce prescribing of multiple potentially harmful medications to older adults in the emergency department, while the study reported by Dreishulte et al 35 focused on the de-implementation of high-risk NSAIDs and antiplatelet agents in primary care. Most interventions (85.2%) aimed to de-implement all types of NSAIDS, although some (14.8%) targeted reduction of a single-type or class of NSAIDs. Interventions largely focused on prescription NSAIDs, with only 7.4% of interventions aimed to reduce potentially harmful OTC NSAIDs. All studies focused on oral NSAIDs; topical NSAIDs were not addressed in any interventions.
More than half of interventions (55.8%) aimed to de-implement NSAIDs classified as high-risk based on patient age (generally > 65 or 70 years), with BEERS, START and STOPP criteria frequently referenced (table 2).36 The de-implementation of potentially harmful NSAIDs among patients with gastrointestinal conditions (eg, peptic ulcer disease and inflammatory bowel disease) or who were taking chronic NSAIDs without gastroprotective medication (eg, proton-pump inhibitor) and kidney disease was also common (47.1% and 38.2%, respectively) (table 2).
Criteria by which the use of non-steroidal anti-inflammatory drugs (NSAIDs) were classified as high-risk or potentially harmful by included studies
Most interventions (76.2%) were effective in reducing the use of high-risk NSAIDs (online supplemental file 3). There was minimal difference in the reported effectiveness of interventions that incorporated an implementation theory/model/framework versus those that did not (68% effective and 78% effective), were clinician versus patient-facing or both clinician and patient-facing (78% effective and 70% effective), single-component versus multi-component (74% effective and 77% effective) and involved only NSAIDs versus multiple medications (76% effective and 71% effective). Effectiveness was similar across intervention types (academic detailing/clinician education (66% effective), clinician financial incentives (79% effective), EHR/clinical decision support (71% effective), patient counselling or education (68% effective), performance feedback (77% effective), pharmacist medication review (70% effective), practice facilitation or coaching (75% effective) or other (80% effective)). A lower proportion of studies taking place in the USA (64% vs 82% for other countries) reported on an intervention that was effective in reducing NSAID utilisation.
Very few studies (5.9%) evaluated patients’ level of pain or quality of life following discontinuation of NSAIDs. Over half of the studies (51.5%) assessed other patient-focused outcomes associated with the interventions, including patient-rated quality of interaction with clinician,37 occurrence of falls38 and emergency department admissions.35
Discussion
Although many professional organisations and societies recommend limiting or avoiding NSAIDs in high-risk patients, potentially harmful prescribing and OTC use persist at undesirable rates.15–18 This scoping review identified 68 studies describing healthcare-based interventions to de-implement potentially harmful NSAIDs published between 1 January 2000 and 31 May 2022. A broad range of intervention types and characteristics were represented, with multi-component, clinician-facing interventions targeting older adults and those with gastrointestinal or renal risk factors in primary care being the most common. This review exposed several knowledge gaps, many of which suggest opportunities for subsequent research, as highlighted below.
Based on the identified research gaps, we suggest several recommendations for future research. First, a more comprehensive analysis of the effectiveness of prior interventions may best inform subsequent interventions to de-implement potentially harmful NSAIDs. The present review identified interventions reported as effective versus not effective in reducing potentially harmful NSAIDs, but, as a scoping review with a stated purpose of overviewing the available literature, did not evaluate effect size, degree of effectiveness or clinical relevance of results. Our evaluation of effective versus not effective interventions yielded few differences with the exception of a greater proportion of not effective interventions reported by US research teams compared with those from other countries. It is unclear to what extent publication bias influenced the reporting of negative outcome interventions, but further efforts to de-implement potentially harmful NSAIDs are needed in the USA, regardless.
Second, we noted that both single-component and multi-component interventions were similarly effective in de-implementing NSAIDs, which is inconsistent with some,32 33 but not all,39 previous literature for reducing the utilisation of low-value health services. In several cases, interventions involving likely low-cost, low-burden approaches (eg, one-time education session, online training modules and pamphlets) were associated with the same reduction in NSAID utilisation as much more elaborate and costly approaches (eg, pharmacist medication review, individual patient counselling and EHR workflow modification). Further research to identify the characteristics of the simplest or most feasible and sustainable interventions is needed, keeping in mind the many contextual variables that influence effectiveness40 and that the effectiveness of intervention components is not additive (ie, a greater number of components in a multi-component intervention is not always better).32
Third, academic detailing and clinician education (most common), performance feedback, pharmacist medication review and EHR modification were frequently evaluated, but several other intervention approaches have been tested less commonly. One option warranting further evaluation is practice facilitation, which leverages external facilitators to employ a variety of practice change strategies and tailor interventions to context. Although more commonly used to implement evidence into practice rather than de-implement practice that is not supported by the evidence,41 there are examples of successful practice facilitation de-implementation interventions.42 43 Direct patient education and counselling were effective for some interventions in the present study44–46 and align with our observation that patient-facing interventions tended to be effective. Engagement with patients can enhance outcomes of deprescribing and other health services interventions47–49 and may be especially germane to the de-implementation of OTC NSAIDs, which were barely addressed by interventions studied. Finally, as many real-world efforts to change clinician behaviour involve financial incentives (eg, insurance pay-for-performance), further evaluation of that approach should be pursued. As the lowest proportion of effective interventions occurred in US studies, considering the unique barriers and facilitators to de-implementation within different health systems is important.
Fourth, as there are many scenarios for which long-term NSAID use may be potentially harmful, interventions focused largely on older adults and those with gastrointestinal or renal risk factors. While these are very important populations to target, their findings are not necessarily generalisable to other patient populations. Despite the need for de-implementing NSAIDs in patients with CVD, heart failure and hypertension,10 11 they were the focus of less than one-third of interventions. Additionally, despite evidence that patients may have limited NSAID literacy,50–53 the issue of duplicate NSAID use was minimally addressed by previous interventions. Thus, moving forward, there are numerous opportunities to focus and tailor de-implementation approaches to the patient populations and contexts where needs exist.
Fifth, outcomes important to patients were inconsistently assessed in the studies reviewed. Despite some evidence that patient satisfaction and trust are not adversely impacted by low-value care de-implementation,54 55 clinicians continue to cite concern about patient response as a predominant de-implementation barrier.24 56–58 In addition to evaluating patient-focused outcomes, future studies should explore the unintended consequences of the interventions. Of the minority of studies that evaluated adverse events or changes in pain following NSAIDs de-implementation, none showed increases in adverse events or pain outcomes.59–63 In fact, one study reported lower pain levels among older adults who reduced NSAIDs as part of a pharmacist review programme.59
Finally, we observed that very few of the identified interventions employed an implementation or de-implementation theory, framework or model. Although there appeared to be no difference in the effectiveness of interventions that did versus did not use such a theory, framework or model, their use facilitates a thorough exploration of factors that led or did not lead to an effective intervention. Furthermore, the use of these theories, frameworks and models can guide successful implementation, adaptation and dissemination of interventions and should be applied in future efforts.64–66 One excellent example is provided by the intervention reported by Pinto et al 67 who included their TIDieR checklist68 in their published manuscript. Future researchers may benefit from categorising interventions based on the 4R’s framework of Norton et al 69 (did the intervention involve removing, replacing, reducing or restricting the inappropriate service?).
This study has some limitations. Our initial search strategy did not comprehensively identify studies that focused on interventions to de-implement NSAIDs as one of the multiple target medications. To incorporate these studies into our review, we added a supplemental secondary database search that was effective in identifying eight additional applicable studies. Although it is possible that this approach may have missed some multiple medication interventions, the process of reviewing reference lists and numerous systematic or scoping review articles was the best available approach for including as many appropriate studies as possible with the resources available. Future literature searches may benefit from the inclusion of ‘polypharmacy’ and associated terms. Additionally, our data extraction plan did not capture whether interventions focused on reducing new prescriptions for (or OTC use of) potentially harmful NSAIDs versus reducing refills for ongoing inappropriate NSAIDs, which could be important to inform future interventions. It is also possible that some authors may have reported data we extracted in separate publications (eg, implementation or de-implementation theory, framework or model). Last, as a scoping review, we did not formally evaluate the quality of the studies reviewed.
Conclusion
This scoping review identified 68 interventions to de-implement potentially harmful NSAIDs published internationally from 1 January 2000 to 31 May 2022. During this time, there was a great deal of evolution in the NSAID market, in the scientific evidence related to the comparative effectiveness and safety of various NSAIDs and other analgesics and in professional recommendations, clinical practice patterns and regulatory policy related to NSAIDs prescribing. Yet, many interventions with varying characteristics were effective in de-implementing potentially harmful NSAIDs during this timeframe. These interventions classified NSAID use/prescribing as high-risk for multiple reasons, employed a variety of de-implementation approaches and took place in several different healthcare settings. We highlight six opportunities to enhance scientific knowledge on NSAID de-implementation interventions in healthcare settings: (1) a comprehensive, systematic analysis of the effectiveness of prior interventions; (2) an evaluation of characteristics and combinations of characteristics associated with highly effective interventions; (3) an assessment of the effectiveness of less-used intervention strategies such as practice facilitation and clinician financial incentives; (4) the evaluation of interventions for varying high-risk patient populations and to de-implement OTC NSAIDs as well as prescription; (5) the inclusion of patient-focused outcomes and (6) the incorporation of implementation or de-implementation theories, frameworks or models to guide the planning, delivery and evaluation of interventions. This subsequent knowledge stands to de-implement a common health service (NSAIDs) and improve medication safety and healthcare quality for a large number of patients living with common health conditions.
Supplemental material
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @MRockwellRD
Contributors MR: study design and strategy, literature review, data extraction, interpretation and preparation of manuscript first draft, guarantor. JE: study design and strategy and interpretation. EGO, ES and JKT: abstract review and data extraction. IY and MV: literature review and interpretation. All authors approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.