Article Text
Abstract
Objectives In trials of acute severe infections or inflammations frequent administration of non-randomised treatment (ie, intercurrent event) in response to clinical events is expected. These events may affect the interpretation of trial findings. Swissped-RECOVERY was set up as one of the first randomised controlled trials worldwide, investigating the comparative effectiveness of anti-inflammatory treatment with intravenous methylprednisolone or intravenous immunoglobulins in children and adolescents with Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS). We present one approach towards improving the interpretation of non-randomised treatment in a randomised controlled trial.
Design This is a pre-planned ancillary analysis of the Swissped-RECOVERY trial, a randomised multicentre open-label two-arm trial.
Setting 10 Swiss paediatric hospitals (secondary and tertiary care) participated.
Participants Paediatric patients hospitalised with PIMS-TS.
Interventions All patient-first intercurrent events, if applicable, were presented to an independent adjudication committee consisting of four international paediatric COVID-19 experts to provide independent clinical adjudication to a set of standardised questions relating to whether additional non-randomised treatments were clinically indicated and disease classification at the time of the intercurrent event.
Results Of 41 treatments in 75 participants (24/41 (59%) and 17/41 (41%) in the intravenous methylprednisolone and immunoglobulin arms of the trial, respectively), two-thirds were considered indicated. The most common treatment (oral glucocorticoids, 14/41, 35%) was mostly considered not indicated (11/14, 79%), although in line with local guidelines. Intercurrent events among patients with Shock-like PIMS-TS at baseline were mostly considered indicated. A significant proportion of patients with undifferentiated PIMS-TS at baseline were not attributed to the same group at the time of the intercurrent event (6/12 unchanged, 4/12 Kawasaki disease-like, 2/12 Shock-like).
Conclusion The masked adjudication of intercurrent events contributes to the interpretation of results in open-label trials and should be incorporated in the future.
Trial registration numbers SNCTP000004720 and NCT 04826588.
- paediatric intensive & critical care
- paediatric infectious disease & immunisation
- post-infectious disorders
- randomized controlled trial
- SARS-CoV-2 infection
Data availability statement
Data are available on reasonable request. Deidentified participant data will be shared on reasonable request unless the request is conflicting with ongoing or planned analyses. Requests need to be addressed to the corresponding author and will require approval by the Swissped-RECOVERY steering group, and with a signed data access agreement. Researchers with a proposed use, approved by appropriate institutional review boards and the Swissped-RECOVERY Steering Committee, can access the data.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- paediatric intensive & critical care
- paediatric infectious disease & immunisation
- post-infectious disorders
- randomized controlled trial
- SARS-CoV-2 infection
STRENGTHS AND LIMITATIONS OF THIS STUDY
These ancillary analyses were pre-planned and the non-randomised events of interest were defined a priori for further evaluation, which resulted in an improvement in the interpretation of the trial findings.
All case narratives were carefully masked not only regarding randomised but also non-randomised anti-inflammatory treatment. Additionally, the time point of the intercurrent event (ICE) was reported as during trial treatment+x hours to avoid unmasking resulting from different durations of treatment administration.
The small sample size and the fact that only patient-first ICE, excluding subsequent ICEs and patients not experiencing ICEs, were adjudicated by the committee is a limitation of the study.
The independent adjudication committee’s reviews occurred in an artificial setting in a virtual meeting and in hindsight which contrasts the clinical bedside decision-making.
Introduction
In trials of acute severe infections or inflammatory syndromes, frequent administration of non-randomised treatment in response to clinical events is expected. In the terminology of the International Council for Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use E9(R1) Addendum on Estimands and Sensitivity Analysis in Clinical Trials, these are defined as intercurrent events (ICEs).1 ICEs take place after randomisation and may affect the interpretation of trial findings. They can be a source of bias if knowledge of allocated treatment differentially affects postrandomisation patient management. The ICH Addendum outlines the importance of explicit preplanned identification and handling of ICEs to enable all clinical questions addressed by a trial to be answered fully and robustly.
Here, we present one approach applied in a recent pragmatic open-label randomised trial (Swissped-RECOVERY) investigating the comparative effectiveness of first anti-inflammatory treatment with intravenous methylprednisolone (IVMP) or intravenous immunoglobulins (IVIG) in children and adolescents with Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS).2 3 Patients with PIMS-TS exhibit clinical and laboratory signs of inflammation together with single or multiple organ dysfunction, in the presence of confirmed or suspected previous exposure to or infection with SARS-CoV-2.3 Overall, the disease presentation was severe in a substantial proportion of children, and even more at the beginning of the pandemic. Therefore, treatment was warranted. However, given that at the time there was no evidence available regarding the best treatment, recommendations were based on expert opinion and consensus guidelines mostly. Corticosteroids and IVIG became the mainstay of treatment informed by the resemblance of PIMS-TS cases and Kawasaki disease. Phenotype classification, that is, Shock-like PIMS-TS, Kawasaki disease-like PIMS-TS and undifferentiated PIMS-TS, emphasising different presentations and severities were routinely considered in the management of PIMS-TS in Switzerland, and therefore, included in our analyses.4 In Swissped-RECOVERY, we expected non-randomised anti-inflammatory treatments to be common and were interested in differentiating between patients experiencing these because of ongoing or progressive inflammation (considered clinically indicated and potentially related to the effectiveness of randomised treatments), and those in whom a clear clinical reason for additional non-randomised anti-inflammatory treatment was lacking. We put in place an independent adjudication committee (IAC) to evaluate these ICEs, masked to randomised and received non-randomised treatment. Here, we describe and interpret the adjudication results, including indicated and non-indicated ICEs and a comparison between randomisation arms.
Methods
Study design
This is a pre-planned ancillary analysis of the Swissped-RECOVERY trial (Swiss National Clinical Trials Portal (SNCTP000004720) and ClinicalTrials.gov (NCT 04826588)), an investigator-initiated randomised multicentre open-label two-arm trial (IVIG vs IVMP) in children hospitalised with PIMS-TS at 10 Swiss paediatric hospitals (Aarau, Basel, Bellinzona, Bern, Fribourg, Geneva, Lausanne, Lucerne, St. Gallen and Zurich).2 We aimed to determine clinical indications of ICEs according to masked IAC consensus as the gold standard.
Patient and public involvement
Given the expedited process of setting up this trial due to the developments of the pandemic, it was not appropriate or possible to involve patients or the public in the design, conduct, reporting or dissemination plans of our research.
Definition of ICEs
ICEs of interest were defined a priori in a dedicated IAC charter (Supplement) as non-randomised anti-inflammatory treatments including additional or fewer doses of the randomised treatment, IVMP in the IVIG group and vice versa, biological treatment, and any oral tapering of glucocorticoids. Patients experiencing at least one of these were presented to the IAC.
Masked IAC
The IAC consisted of four international PIMS-TS experts who met virtually in five sessions between 6 June 2022 and 9 August 2022. The work of the IAC was governed by a dedicated charter (online supplemental file 1), and in line with this, at least two members had to be present at each meeting. All chronologically first ICEs per patient were assessed, meaning if one patient experienced multiple ICEs, the clinical indication was adjudicated only for the first non-randomised anti-inflammatory treatment. Masked narratives were prepared and presented by a non-independent facilitator (TW), who did not contribute to the discussions about clinical indication but provided further information on IAC request. IAC consensus decisions were required by the agreement of all present experts and were recorded directly into a designated form on the electronic data capture system REDCap.
Supplemental material
Configuration of ICE narratives
The case narratives presented to the IAC included baseline general information (patient demographics, known exposure to a SARS-CoV-2 case, estimated number of weeks since SARS-CoV-2 exposure and underlying comorbidities), clinical characteristics (organ involvement, vital signs, need for inotropes, respiratory support or fluid resuscitation), cardiological examinations (ECG, echocardiogram), laboratory parameters (SARS-CoV-2 PCR and serology, haematology, coagulation and biochemical markers) and follow-up information for these variables until the ICE. All narratives were carefully masked regarding randomised treatment and non-randomised treatment received. The time point of the ICE was shown as ‘during trial treatment+x hours’ to avoid unmasking resulting from the differential duration of IVIG (one dose) and IVMP (one daily dose for three consecutive days).
Adjudication details
The IAC adjudicated ICEs starting with disease classification at the time of the ICE, defined as in the Best Practice Recommendations for the Diagnosis and Management of PIMS-TS in Switzerland4: (1) Shock-like PIMS-TS, (2) Kawasaki disease-like PIMS-TS, (3) undifferentiated PIMS-TS and (4) other disease; in case of (5), no further adjudication was required. The IAC was aware of the site investigator’s allocation at baseline but not at the time of the ICE. For (1–3), the first question was followed by the likelihood that the ICE was clinically indicated: (1) definitely >80%, (2) probably 51%–80%, (3) unlikely 21%–50%, (4) not <21%, (5) too little information. ICEs classified as (5) were represented to the IAC on receipt of additional narrative information. ICEs considered to be in category (1) or (2) were classified as ‘clinically indicated’.
Statistical analysis
Exploratory description of baseline patient characteristics was summarised using the number (percentage) for categorical variables and the median (IQR) for continuous variables. Between-group differences were investigated using the χ2 test for categorical variables and the Wilcoxon rank sum test for continuous variables. Due to the small number of patients and skewed data, parametric testing was not appropriate.
A statistical significance level of 5% was considered statistically significant throughout. All analyses were performed in R (V.4.2.2).5
Results
Between 21 May 2021 and 15 April 2022, a total of 76 patients were enrolled. Of these, 75 patients were included in the primary analysis (37 were allocated to IVMP and 38 were allocated to IVIG). Detailed information on the cohort, including baseline characteristics, is presented in the original publication.6
Non-randomised anti-inflammatory treatment
In total, 41 ICEs were adjudicated. In the IVMP arm, 24/37 (65%) patients experienced at least one ICE, compared with 17/38 (45%) in the IVIG arm (p=0.13).
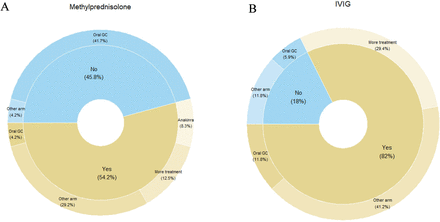
The most common first ICE was oral glucocorticoids, with or without tapering, accounting for 14/41 (34%) ICEs (11/24 (46%) in the IVMP and 3/17 (18%) in the IVIG arm). Further first ICEs occurred because of the addition of non-randomised treatment, including IVMP >3 days or >10 mg/kg; IVMP in case of IVIG randomisation or vice versa, IVIG >2 g/kg or >1 dose and intravenous or subcutaneous anakinra administration (figure 1).
(A) A total of 24 ICEs reported; considered clinically indicated 13/24, with administration of IVIG in 7/13 clinically indicated ICEs; considered non-indicated 11/24, with administration of oral glucocorticoids in 10/11 non-indicated ICEs. (B) A total of 17 ICEs reported; considered clinically indicated 14/17 with administration of IVMP in 7/14 clinically indicated ICEs; considered non-indicated 3/17. GC, glucocorticoids; ICE, intercurrent event; IVIG, intravenous immunoglobulins; IVMP, intravenous methylprednisolon.
IAC findings
Non-randomised anti-inflammatory treatment was considered clinically indicated by the IAC for 27/41 (66%) patients (13/24 (54%) in the IVMP arm, 14/17 (82%) in the IVIG arm). Overall, there was a trend towards a greater proportion of clinically indicated ICEs among patients in the IVIG arm (p=0.061). Non-indicated ICEs in the IVMP arm were dominated by receipt of oral glucocorticoids (10/11; 91%). Non-indicated ICEs were rare in the IVIG arm (3/17; 18%) and comprised in two cases of switch to IVMP and in one case of addition of oral glucocorticoids (figure 1).
A different pattern of ICEs and their clinical indication was observed among patients with the three phenotypes of PIMS-TS (table 1). ICEs among patients with Shock-like PIMS-TS at baseline were mostly considered indicated. For patients with Kawasaki disease-like PIMS-TS at baseline, 7/8 ICEs among patients randomised to IVIG were considered indicated, in contrast to only 2/6 among patients randomised to IVMP. ICEs were more common among patients with undifferentiated PIMS-TS at baseline and allocated to IVMP (10/12) compared with IVIG (2/12). Of note, while patients considered to show a Shock-like or Kawasaki disease-like clinical phenotype at baseline most displayed the same phenotype at the time of receipt of non-randomised anti-inflammatory treatment (12/15 Shock-like patients, 11/14 Kawasaki disease-like patients), this was not the case for the undifferentiated PIMS-TS group (6/12 unchanged, 4/12 Kawasaki disease-like at time of ICE, 2/12 Shock-like) (figure 2).
Patients considered to show a Shock-like or Kawasaki disease-like clinical phenotype of PIMS-TS at baseline most displayed the same phenotype at the time of receipt of non-randomised anti-inflammatory treatment (12/15 Shock-like PIMS-TS, 11/14 Kawasaki disease-like PIMS-TS), this was not the case for the undifferentiated PIMS-TS group (6/12 unchanged, 4/12 Kawasaki disease-like, 2/12 Shock-like at the time of the ICE). ICE, intercurrent event; PIMS-TS, Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2.
Independent masked adjudication of intercurrent events of additional anti-inflammatory treatment according to three clinical phenotypes of Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS)
Clinical and laboratory characteristics of patients with ICEs
Whereas there was a difference in baseline characteristics for patients with and without ICEs in lymphocytopaenia, thrombocytopaenia, ferritin, D-dimers and need for inotropic support, no such difference was observed when comparing baseline characteristics of patients with a clinically indicated versus non-indicated ICE, apart from a longer fever duration in patients with a clinically indicated ICE (table 2).
(A and B) Baseline characteristics stratified by the presence or absence of an ICE (A) and stratified by the IAC consensus (B)
Discussion
Swissped-RECOVERY was the first research group to publish data from a randomised controlled trial on medical interventions in patients with PIMS-TS investigating treatment response to just one immunomodulatory treatment (IVMP compared with IVIG). Masked end-point review committees have been used in open-label trials to mitigate against bias in endpoint assessment.7 8 Analogously, we involved an IAC to provide independent adjudication on the necessity/indication for non-randomised anti-inflammatory treatments, given that their clinically indicated use may reflect limitations in effectiveness of the first randomised treatment.
While we did not identify a relevant difference in effectiveness between the first treatment with IVMP or IVIG in the main trial analysis taking a standard intention-to-treat approach,6 we noted the high proportion of participants receiving non-randomised anti-inflammatory treatment (41/75, 55%). With 55% of patients receiving non-randomised anti-inflammatory treatment, there is a risk of many patients converging on a single treatment or being exposed to both treatments, reducing the informativeness of the trial. The IAC considered two out of three of these ICEs clinically indicated, mostly in children presenting with Shock-like PIMS-TS patients and in those with Kawasaki disease-like PIMS-TS when allocated to IVIG. However, the IAC also identified one in three ICEs as not clinically indicated. Those ICEs predominantly comprised added oral glucocorticoids. This assessment supports the conclusion that monotherapy with either IVMP or IVIG is sufficient and safe for the majority of the study population (48/75, 64%; 34 patients with no ICE and 14 patients with a clinically non-indicated ICE) but may need to be expanded in critically unwell patients not responding to treatment after a period of observation. Our findings specifically highlight that the addition of a tapering regimen of oral corticosteroids after a course of IVMP4 9 seems to be largely unnecessary.
Disease classification and severity influence adjudication and clinical decision-making, leading to non-randomised treatment usually being considered indicated among patients with Shock-like PIMS-TS. PIMS-TS is difficult to distinguish from Kawasaki disease. IVIG is the standard treatment for Kawasaki disease10 and so may have been added to the allocated treatment in a proportion of patients randomised to IVMP, due to investigator concern about undertreating possible Kawasaki disease. Such non-randomised treatment was usually considered non-indicated. ICEs that were identified as non-indicated may reflect variability in regional practice and evolution of local, national and international guidelines during the trial, such as tapering of oral corticosteroids4 (predominately related to existing recommendations for the treatment of Kawasaki disease9).
IAC interpretation of ICEs in Swissped-RECOVERY had several limitations. First, narratives had to be presented in a way that prevented inferences on allocated treatment and unmasking of the exact nature of the ICE. This limited information available to the IAC, potentially impacting their adjudication. Second, IAC reviews rely on the clinical expertise of independent members. Since PIMS-TS was an emerging disease at the time of the trial, the IAC members had limited evidence available to inform management, potentially leading to more permissive adjudication relying on experience and expertise alone. Furthermore, IAC reviews occur in a somewhat artificial setting where experts adjudicate ICEs in a virtual meeting in contrast to clinicians making bedside decisions. Fourth, only patients’ first ICEs were reviewed. A review of all ICEs may have provided further insight into the management of PIMS-TS patients in the trial but would have substantially increased the complexity of the review process. Fifth, the IAC was not asked to adjudicate the management of patients not experiencing ICEs. This may theoretically have identified patients who should have, in the view of the IAC, received additional anti-inflammatory treatment, adding to the interpretation of trial findings. Lastly, the analyses considering phenotype classification rely on the classification at baseline. However, especially for undifferentiated PIMS-TS, there is a substantial proportion of cases being reclassified by the IAC, which might further impact the interpretation of the results.
We considered rapid reporting of primary and secondary endpoints from an interventional randomised controlled trial in PIMS-TS, an emerging disease with a potentially high global impact, as an utmost priority. IAC review can be complex and needs to be carefully prepared and supported by the trial team to maintain the masking of adjudicating members. We, therefore, took the decision to present the trial findings within a standard intention-to-treat framework but incorporated the IAC review in our statistical analysis plan as a key secondary analysis to address and robustly interpret the expected high frequency of non-randomised anti-inflammatory treatment.
Overall, IAC reviews proved valuable in providing an independent assessment of whether non-randomised anti-inflammatory treatment was likely given as treatment for persistent or progressive PIMS-TS. This was found to have been the case in two out of three ICEs considered. Alternative or complementary strategies to minimise clinically non-indicated deviations from randomised treatment would be the utilisation of sequential randomisation as well as rigorous training and increased documentation requirements for ICEs. Neither of these strategies would have been compatible with the pragmatic nature of the trial. We, therefore, feel that IAC assessments should be considered in the context of the Estimand Framework in future open-label trials, as the information can be incorporated into prespecified analyses and will help to improve the interpretation of trial findings.
Data availability statement
Data are available on reasonable request. Deidentified participant data will be shared on reasonable request unless the request is conflicting with ongoing or planned analyses. Requests need to be addressed to the corresponding author and will require approval by the Swissped-RECOVERY steering group, and with a signed data access agreement. Researchers with a proposed use, approved by appropriate institutional review boards and the Swissped-RECOVERY Steering Committee, can access the data.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the lead ethics committee (Ethics Committee Northwest and Central Switzerland, EKNZ, Project ID: 2021-00362) and other responsible ethics committees in Switzerland (ie, Bern, Geneva, Eastern Switzerland, Ticino, Vaud and Zurich). Written informed consent has been obtained.
Acknowledgments
The study team would like to express their gratitude to all parents and children participating in this study. In addition, the authors are grateful to all study team members involved in the study conduct across the sites and SwissPedNet for the support. The authors thank Dr Alasdair Bamford from the Great Ormond Street Hospital for Children, London, England; Dr Kate Webb from the South African College of Paediatrics, Paediatric Rheumatology, South Africa; Dr Pablo Rojo from the University Hospital 12 October Madrid, Spain; and Dr Andriana Tremoulet from the Rady Children’s Hospital San Diego, US for their participation in the blinded independent adjudication committee. Furthermore, the authors thank Dr Michelle Clements from the MRC Clinical Trials Unit at UCL, London, England; Professor Dr Carlo Giaquinto from the University of Padova, Italy; and Dr Robin Kobbe from the University Medical Center Hamburg-Eppendorf, Institute for Infection Research and Vaccine Development (IIRVD), and Department of Infectious Disease Epidemiology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany for their participation in the independent data monitoring committee.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
NS and CS contributed equally.
Presented at These data have been submitted as an abstract and accepted as a poster at the Annual Meeting of the European Society of Paediatric Infectious Diseases (ESPID) in Lisbon 2023.
Collaborators Maya C Andre, MD, PhD, Division of Respiratory and Critical Care Medicine, University Children’s Hospital Basel, University of Basel, Basel, Switzerland, Douggl G N Bailey, MD, Paediatric and Neonatal Intensive Care Unit, Children’s Hospital of Eastern Switzerland, St. Gallen, Switzerland, Geraldine Blanchard-Rohner, MD, Paediatric Immunology and Vaccinology Unit, Division of General Paediatrics, Department of Child, Woman and Adolescent Medicine, Faculty of Medicine, Geneva University Hospitals, Geneva, Switzerland, Michael Buettcher, MD, Paediatric Infectious Diseases Unit, Department of Paediatrics, Cantonal Hospital Lucerne, Lucerne, Switzerland, Serge Grazioli, MD, Division of Neonatal and Paediatric Intensive Care, Department of Child, Woman and Adolescent Medicine, Faculty of Medicine, Geneva University Hospitals, Geneva, Switzerland, Henrik Koehler, MD, MHBA, Department of Paediatrics, Cantonal Hospital Aarau, Aarau, Switzerland, Maria-Helena Perez, MD, Paediatric Intensive Care Unit, Lausanne University Hospital and Lausanne University, Lausanne, Switzerland, Johannes Trück, MD, DPhil, Division of Immunology and Children’s Research Center, University Children’s Hospital Zurich, University of Zurich, Zurich, Switzerland, Federica Vanoni, PD, Institute of Paediatric of Southern Switzerland, Ente Ospedaliero Cantonale, Bellinzona, Switzerland, Petra Zimmermann, MD, PhD, Department of Paediatrics, University of Fribourg and Fribourg Hospital, Fribourg, Switzerland acted as PIs at local sites, performed patient recruitment, data collection and approved the final version of the manuscript.
Contributors JAB, TW and CS planned and implemented the masked review of intercurrent events. JAB and NS contributed to the first draft, approved the final version and took responsibility for the accuracy of the reported findings. JAB acts a guarantor. AB, KW, AT, PR, TW, LJS, AA and CS contributed to the draft and approved the final version. JAB acts as guarantor. CS performed the analysis and is the data manager for Swissped-RECOVERY.
Funding This work was supported by grants from the NOMIS Foundation, the Vontobel Foundation and the Gaydoul Foundation (LJS). Swiss PedNet (https://www.swisspednet.ch/) provides infrastructure support for study coordination, Good Clinical Practice and monitoring.
Competing interests JAB received grant support paid to the institution from the European and Developing Countries Clinical Trials Partnership (PediCaP, RIA2017MC-2023), Horizon 2020 (NeoIPC, grant 965328), the Swiss National Science Foundation (KIDS-STEP, grant 173532), National Institute for Health Research (CAP-IT, project 13/88/11), Innosuisse (SPEARHEAD flagship grant), the Swiss Personalised Health Network (Secretariat for Education Research and Innovation) (SwissPedHealth, award NDS-2021-911), in the past 36 months; consulting fees paid to the institution from Shionogi, Sandoz, Basilea, and GSK; payments to the institution for presentations, lectures, speakers bureaus, manuscript writing or educational events in the past 36 months from Pfizer, Sandoz and Bayer; participated at independent data monitoring committee boards of Avenir trial (member, expenses), Lakana trial (member, unfunded), CURLY trial (Chair, unfunded) in the past 36 months; is the vice president of the SwissPedNet (unpaid) and leadership of Severe Bacterial Infection and Antimicrobial Resistance working group of the Penta Foundation (unpaid). TW gave presentations for Novartis (payment to the institution) in the past 36 months. AB had received fixed-term consultancy fees from Gilead. KW is supported by the Crick African Network (CAN). The CAN receives its funding from the UK’s Global Challenges Research Fund (MR/P028071/1), the Francis Crick Institute which receives its core funding from Cancer Research UK (FC1001647), the UK Medical Research Council (FC1001647) and the Wellcome Trust (FC1001647). KW is also supported by the South African Medical Research Council with funds received from the National Treasury. PR received grant support from ViiV and consulting fees from MSD. AT received grant support paid to the institution/UCSD from the National Institute of Health and consulted Janssen Pharmaceuticals and Kiniksa with no payment received. All other authors declared no conflicts of interest.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.