Article Text
Abstract
Objectives This study aimed to assess the appropriateness of prescribing profiles and intake adherence to non-vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation (AF).
Design Retrospective longitudinal study.
Setting The study was conducted in the Regional Health Administration of Northern Portugal.
Participants The authors selected a database of 21 854 patients with prescriptions for NOACs between January 2016 and December 2018 and were classified with AF until December 2018.
Outcome measures The appropriate dosage of NOAC for patients with AF divided into three categories: contraindicated, inconsistent and consistent, based on the 2020 European Society of Cardiology guidelines for AF.
Results Dabigatran had a lower percentage of guideline-consistent doses (n=1657, 50.1%) than other drugs such as rivaroxaban (n=4737, 81.6%), apixaban (n=3830, 78.7%) and edoxaban (n=436, 82.1%). Most patients with an inconsistent dose were prescribed a lower dose than recommended based on their glomerular filtration rate (GFR). Among patients younger than 75 years with GFR >60 mL/min, 59.8% (n=10 028) had an adequate GFR range, while 27.8% (n=7166) of GFR measurements from patients older than 75 years old and 29.4% (n=913) of GFR measurements from patients younger than 75 years with GFR <60 mL/min were within an adequate time range. Adherence to NOACs varied across different drugs, with 59.1% (n=540) adhering to edoxaban, 56.3% (n=5443) to rivaroxaban, 55.3% (n=3143) to dabigatran and 53.3% (n=4211) to apixaban.
Conclusions Dabigatran had the lowest percentage of guideline-consistent doses. Patients younger than 75 years with GFR >60 mL/min had the highest percentage with an adequate GFR range, while other groups who require closer GFR monitoring had lower percentages within an adequate GFR range. Adherence to NOACs differed among different drugs, with greater adherence to treatment with edoxaban and less adherence to apixaban.
- Stroke
- Primary Health Care
- Anticoagulation
Data availability statement
No data are available. Not applicable.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
All non-vitamin K antagonist oral anticoagulant (NOAC) dispensations were evaluated, regardless of whether the prescription was issued by a family doctor, hospital doctor from the National Health Service or doctor from a private institution, for evaluation of adherence to NOAC intake among patients with atrial fibrillation.
The main limitation of this real-world study is possible registration and codification bias, as the data are obtained from electronic health records created by family doctors.
When assessing the appropriateness of the prescribed NOAC dose, only the most recent glomerular filtration rate, weight and age criteria were considered.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is frequently associated with chronic kidney disease (CKD).1 The first part of the AF-React Study aimed to determine the prevalence of AF and to assess how these patients are being cared for: what anticoagulants are being prescribed and are they being prescribed as recommended. The renal function analysis revealed that 41.1% of 63 526 patients with AF had a glomerular filtration rate (GFR) of ≤60 mL/min.2 There is an intimate relationship between AF and CKD. On the one hand, kidney-specific mechanisms can alter the cardiac structure and predispose it to AF. On the other hand, the development of AF can accelerate the progression of CKD.1 As with the general population, AF in patients with CKD is associated with an increased risk of thromboembolism and stroke.3 The synergistic effect of these two conditions raises serious issues concerning the balance between bleeding and thrombotic risk. Anticoagulant treatment can be challenging, especially in stage 5 CKD, where the clinical benefit is still unclear.1
Oral anticoagulation (OAC) is the most effective form of thromboprophylaxis in patients with AF with an increased risk of stroke. However, reducing stroke risk is directly related to the appropriateness of OAC prescription and adherence to OAC intake in these patients.4
Previous studies have shown that non-vitamin K antagonist oral anticoagulants (NOACs) such as dabigatran, rivaroxaban, apixaban and edoxaban are superior to warfarin in preventing thromboembolic events in patients with non-valvular AF, providing increased safety and a reduction in the number of bleeding events overall.5–8 Therefore, they are currently recommended for patients with AF at risk of stroke after calculating the CHA2DS2-VASc score.9
However, the metabolism of NOACs largely depends on the kidneys for elimination, and patients with creatinine clearance (CrCl) <25 mL/min, who were excluded from all phase III NOAC trials, are not well studied.3
The 2020 European Society of Cardiology guidelines9 and 2021 European Heart Rhythm Association (EHRA) practical guide10 recommend dose adjustment for apixaban, rivaroxaban and edoxaban in stage 4 CKD and do not recommend the use of dabigatran. For GFR ≤50 mL/min, dose adjustment is recommended for all NOACs.
This is particularly relevant since the kidneys are responsible for partially eliminating all four available NOACs. Dabigatran has the greatest extent of renal elimination (80%), while 50%, 35% and 27% of edoxaban, rivaroxaban and apixaban, respectively, are cleared via the kidneys.11
Other conditions also influence the appropriate prescription of NOACs in patients with AF. For dabigatran, a reduced dose should be prescribed if the patient is over 80 years old, has concomitant administration with verapamil or has an increased risk of bleeding. For apixaban, a reduced dose is recommended for patients over 80 years old or with a body weight of <60 kg. For edoxaban, a reduced dose is recommended for patients with a body weight of <60 kg or with concomitant administration of dronedarone, ciclosporin, erythromycin or ketoconazole.10
Lowres et al identified various factors associated with poor adherence to OAC intake. Medical factors include no history of stroke/transient ischaemic attack or low stroke risk, fewer comorbidities, high bleeding risk, paroxysmal AF, lack of AF symptoms, electrical cardioversion after commencing OAC and taking ≥2 dosages per day. Patient factors include younger age, lower health literacy, low AF knowledge, unawareness of associated stroke risk, poor OAC knowledge, concerns about bleeding and lifestyle changes, information overload, anger, depression or anxiety from the AF diagnosis, low treatment satisfaction, busy work schedule, lack of health insurance coverage and low ability to pay for medications.4
This study aims to assess the appropriateness of the prescribing profile and adherence to the intake of NOACs in patients with AF.
Methods
Data collection
This study was performed using the AF-React Project database. The Department of Studies and Planning within the Regional Health Administration of Northern Portugal developed the AF-React Project database, which includes data from 2016, 2017 and 2018. To identify patients with AF, the International Classification of Primary Care, second edition (ICPC-2) code K78 for AF was used.
The AF-React Study encompasses all adults (18 years or older) whose clinical records in primary healthcare, under the purview of the Regional Health Administration of Northern Portugal, included the K78 code prior to December 2018. For the purpose of this study, we focused on individuals who were prescribed the same NOAC (novel oral anticoagulant) within the study period of 2016–2018. So, a retrospective longitudinal study was conducted.
NOAC dosage
In order to investigate the appropriate dosage of NOAC for patients with AF, we classified patients into three dosage categories: contraindicated, inconsistent and consistent with the guidelines. The patient’s most recent GFR value was considered when making this classification.
Regarding dabigatran,10 a GFR <30 mL/min was classified as contraindicated for use. Inconsistent with the guidelines dosage status was assigned if (1) the patient was not prescribed a 110 mg dose when the GFR was between 50 and 30 mL/min, or a 150 mg dose when the GFR was above 50 mL/min, or (2) the patient was over 80 years old and not prescribed a 110 mg dose. Consistent with the guidelines dosage status was assigned if (1) the patient was prescribed a 110 mg dose when the GFR was between 50 and 30 mL/min, or a 150 mg dose when the GFR was above 50 mL/min, and (2) the patient was over 80 years old and prescribed a 110 mg dose with a GFR above 30 mL/min.
For rivaroxaban,10 a GFR <15 mL/min was classified as contraindicated for use. Inconsistent with the guidelines dosage status was assigned if the patient was not prescribed a 15 mg dose when the GFR was between 50 and 15 mL/min or a 20 mg dose when the GFR was above 50 mL/min. Consistent with the guidelines dosage status was assigned if the patient was prescribed a 15 mg dose when the GFR was between 50 and 15 mL/min or a 20 mg dose when the GFR was above 50 mL/min.
For apixaban, a GFR <15 mL/min was classified as contraindicated for use. Inconsistent with the guidelines dosage status was assigned if (1) the patient was not prescribed a 2.5 mg dose when the GFR was between 30 and 15 mL/min, or a 5 mg dose when the GFR was above 30 mL/min, or (2) the patient was over 80 years old or weighed less than 60 kg and not prescribed a 2.5 mg dose of apixaban. Consistent with the guidelines dosage status was assigned if (1) the patient was prescribed a 2.5 mg dose when the GFR was between 29 and 15 mL/min, or a 5 mg dose when the GFR was above 30 mL/min, or (2) the patient was over 80 years old or weighed less than 60 kg, had a GFR above 15 mL/min and was prescribed a 2.5 mg dose of apixaban.
For edoxaban, a GFR <15 mL/min was classified as contraindicated for use. Inconsistent with the guidelines dosage status was assigned if (1) the patient was not prescribed a 30 mg dose when the GFR was between 50 and 15 mL/min, or a 60 mg dose when the GFR was above 50 mL/min, or (2) the patient weighed less than 60 kg and was not prescribed a 30 mg dose of edoxaban. Consistent with the guidelines dosage status was assigned if (1) the patient was prescribed a 30 mg dose when the GFR was between 50 and 15 mL/min, or a 60 mg dose when the GFR was above 50 mL/min, or (2) the patient weighed less than 60 kg, had a GFR above 15 mL/min and was prescribed a 30 mg dose of edoxaban.
Monitoring renal function and prescribing NOAC
Regarding the monitoring of renal function and prescribing NOAC, the analysis was divided into three groups: patients over 75 years old (monitored every 6 months), patients 75 years old or younger with a GFR over 60 mL/min (monitored annually), and patients 75 years old or younger with a GFR under 60 mL/min (monitored according to the most recent GFR measurement). For each group, the number of patients with a GFR within the appropriate range (within 0 months or earlier), a GFR up to 6 months after the appropriate range and a GFR after 6, 12 or 24 months after the appropriate range is provided.
Adherence to NOAC
To examine adherence to NOAC intake among patients with AF who received the same NOAC during the study period, all NOAC dispensations were evaluated, regardless of whether the prescription was issued by a family doctor, hospital doctor from the National Health Service or doctor from a private institution. Patients were considered adherent if they received at least 90% of the prescribed pills from the time of the AF diagnosis or the beginning of the study until the end.12
Data analysis
The categorical variables were described using absolute and relative frequencies, n (%). The statistical analysis was performed using the software R.13
Patient and public involvement
None.
Results
Data description
Out of a total of 63 526 patients diagnosed with AF/atrial flutter (ICPC-2 code: K78) in the northern region of Portugal until December 2018, we identified 21 854 patients who were prescribed NOACs during the study period. These NOACs included dabigatran (5219 prescriptions), rivaroxaban (8801 prescriptions), apixaban (7052 prescriptions) and edoxaban (782 prescriptions). Understanding the temporal relationship between NOAC prescription and K78 coding is crucial to determine whether NOACs were prescribed after AF diagnosis. Of the 21 455 (98%) patients, 188 780 prescriptions were issued after the K78 code.
In the first part of the AF-React Study, a primary limitation was identified: the assessment of AF was based on data coded in the clinical process in primary healthcare. Therefore, in this analysis, we examined how codification has evolved over the years (see figure 1). Our findings indicate that the frequency of ICPC-2 K78 coding has increased over time. Additionally, since 2015, the number of AF diagnoses coded has decreased, suggesting that from this date, the ICPC-2 K78 coding is more closely related to new and accurate diagnoses rather than the detection of previous diagnostic coding errors.
ICPC-2 K78 codification by year. ICPC-2, International Classification of Primary Care, second edition.
NOAC dosage
The appropriate dosage of NOAC is critical for anticoagulation to effectively prevent stroke. To evaluate the prescribed dose, we analysed 128 603 prescriptions from 16 282 patients with at least one GFR value (table 1).
The prescribed dose of non-vitamin K antagonist oral anticoagulants
Approximately 89.1% (14 507) of patients maintained the same dose status: 19 were contraindicated, 10 660 were consistent with the guidelines and 3828 were inconsistent with the guidelines. Notably, dabigatran had fewer doses consistent with the guidelines than other drugs: dabigatran (50.1%), rivaroxaban (81.6%), apixaban (78.7%) and edoxaban (82.1%). Most patients with a dose inconsistent with the guidelines were on a lower dose than recommended based on their GFR: dabigatran (46.5%), rivaroxaban (11.7%), apixaban (20.0%) and edoxaban (9.4%). Among patients with a dose inconsistent with the guidelines due to weight and age, those on dabigatran who were over 80 years of age were prescribed a dose of 150 mg (27 patients) or 75 mg (61 patients); those on apixaban who were over 80 years of age or weighed less than 60 kg were prescribed a dose of 5 mg (47 patients); and those on edoxaban who weighed less than 60 kg were prescribed a dose of 60 mg (18 patients).
Monitoring renal function and prescribing NOAC
Another crucial aspect of NOAC therapy to effectively prevent stroke is adequate renal monitoring by physicians. After analysing renal function monitoring, we found that 19 877 patients had at least one GFR measurement during the 3-year study, resulting in 45 679 records. Of these, 25 819 (56.5%) measurements were from 10 792 (54.3%) patients over 75 years old, while 16 757 (36.7%) GFR records were from 8469 (45.4%) patients younger than 75 years old with GFR levels above 60 mL/min. Only 1737 (8.7%) patients 75 years or younger had 3103 (6.8%) GFR values at most 60 mL/min.
Regarding the appropriate GFR ranges for monitoring renal function and prescribing NOAC, we found that (1) 7166 (27.8%) GFR measurements from patients over 75 years old, (2) 10 028 (59.8%) GFR measurements from patients under 75 years old with GFR levels above 60 mL/min, and (3) 913 (29.4%) measurements from patients under 75 years old with GFR levels below 60 mL/min fell within the recommended time range. However, many GFR measurements did not have an adequate range. For those with an inadequate range of GFR, we documented the number of months beyond the recommended range, which is described in table 2.
Analysis of ranges between GFR measurements for monitoring renal function for prescribing non-vitamin K antagonist oral anticoagulants in three groups: patients >75 years old, patients ≤75 years old with GFR >60 mL/min and patients ≤75 years old with GFR <60 mL/min
Adherence to NOAC
To analyse adherence to NOAC intake in patients with AF, we considered the NOAC dispensed due to adherence to therapy, regardless of whether the patient had a medical prescription for the medication.
Regarding dispensation analysis, 24 426 patients were dispensed the same NOAC at the pharmacy during the study period. When we examined the relationship between dispensation and the K78 coding date, we found that 5837 patients were dispensed 25 890 NOACs before the K78 coding date, while 24 164 patients were dispensed 295 551 NOACs after the K78 coding date. Additionally, 5576 patients were dispensed the same NOAC before and after the AF/atrial flutter coding. Of the 24 164 patients who were dispensed NOACs after the K78 coding date, 5683 (23.5%) were dispensed dabigatran, 9667 (40.0%) were dispensed rivaroxaban, 7901 (32.7%) were dispensed apixaban and 913 (3.8%) were dispensed edoxaban.
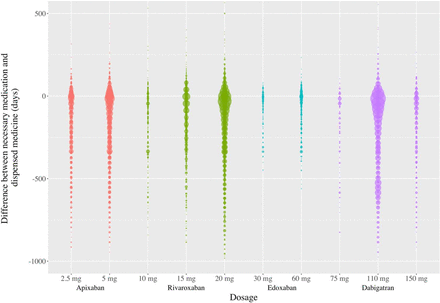
Figure 2 shows the difference between the number of pills required to complete the therapy and the number of pills actually dispensed.
The difference between the number of pills needed to complete the therapy and the number of pills dispensed.
Based on this analysis, there was greater adherence to treatment with edoxaban and less adherence to treatment with apixaban. Patients who were considered adherent had received at least 90% of the pills intended to be taken from the time of diagnosis of AF or the start of the study until the end.12 Approximately 59.1% of patients adhered to edoxaban, 56.3% to rivaroxaban, 55.3% to dabigatran and 53.3% to apixaban. Furthermore, we compared the adherence to NOAC of patients who were diagnosed with AF (K78) before the start of the study period with the adherence of those who were diagnosed with AF (K78) after the study: apixaban (44.1%, 57.1%), dabigatran (56.0%, 54.0%), edoxaban (8.1%, 67.1%) and rivaroxaban (50.7%, 60.2%).
We also analysed which patients had received all the pills necessary to complete the entire treatment during the study period: edoxaban (29.4%), rivaroxaban (24.3%), apixaban (22.6%) and dabigatran (18.5%).
Discussion
Summary
Dabigatran has the lowest percentage of doses consistent with the guidelines, with 46.5% of patients being medicated with a lower dose inconsistent with the guidelines. Patients who are 75 years old or younger, have a GFR greater than 60 mL/min and whose renal function should be monitored annually have the highest percentage of an adequate range of GFR. Other groups who require closer GFR monitoring have a lower percentage of an adequate range of GFR. Adherence to NOACs varies with different drugs: there was greater adherence to treatment with edoxaban and less adherence to apixaban.
Comparison with existing literature
Antunes et al evaluated the prescription of OAC in four family health units in Northern Portugal from January 2010 to December 2015. They found that 76.7% of patients diagnosed with AF based on ICPC-2 coding were medicated with OAC.14 However, in the period from 2016 to 2018, there was an increase in prescriptions to 98%. This suggests an improvement in the anticoagulation of patients with AF in the northern region of Portugal over the years. These results contradict some European studies that showed resistance from family physicians to introduce anticoagulation after the diagnosis of AF,15–18 and contradict the findings of the study by Turakhia et al, which showed that the medical specialty that diagnoses AF influences the decision to anticoagulate or not.19
The electronic clinical files of primary healthcare contain a list of health problems for which there is a follow-up plan, relevant diseases and those who require continuous medical treatment. Accurate records ensure the adequacy of care and enable monitoring and evaluation of the care provided to the population.20 The use of the ICPC-2 is essential for this purpose. Data published in 2015 by the Central Administration of the Health System (CAHS) showed a growing codification of health problems at a national level, reflecting the increase in computerised clinical records and demand from users and healthcare providers.21 This study found that the ICPC-2 K78 encoding has become more frequent over the years, which is consistent with the CAHS data at the national level. In 2011, 20.6 million health problems were identified, and by 2013, this figure had increased to 30.2 million. The percentage of consultations with ICPC-2 coding in primary healthcare is high in Portugal (69.2% in 2011, 83.9% in 2012 and 84% in 2013).21
Sugrue et al found that NOAC dosing was inconsistent with the guidelines in 14.8% of patients at the Mayo Clinic: 12.4% received an inappropriate lower dose and 2.4% received a higher dose inconsistent with the guidelines.22 Even the ORBIT-AF II Registry, a nationwide AF registry conducted in a community practice in the USA, showed that a dose of NOAC inconsistent with the guidelines was prescribed in only 12.5% of cases: underdosing in 9.3% of patients and overdosing in 3.3% of patients, respectively.23 However, a real-world registry in Spain reported higher rates of underdosing and overdosing on NOAC therapy: 17.5% and 14.9%, respectively.24 This study’s results agree with those of the Mayo Clinic and ORBIT-AF II Registry Studies, with underdosing in 15.1% and overdosing in 1.8% of patients. These findings are consistent with studies conducted in other countries. Similar to studies carried out in other countries, in Portugal, taking into account that this study included patients from primary healthcare in the northern region, the same prescription profile can be considered throughout the entire National Health Service. So, due to the risks associated with prescribing inconsistent with the guidelines, greater attention is needed from Portuguese family doctors, emphasising the need for collaboration with health planners to implement a medical educational agenda. This agenda aims to enhance the knowledge and practices related to anticoagulation, possibly addressing issues such as proper prescription, monitoring and management of anticoagulant therapy.
Stamellou and Floege highlighted the importance of regular checks of renal function in patients receiving NOACs to avoid overdosing, especially in situations that may cause acute-on-chronic kidney injury. In such patients, apixaban may be the safest licensed NOAC because of its relatively low renal elimination. In more advanced CKD, that is, stage 4 and particularly in stage 5, NOACs are not recommended due to the lack of randomised controlled trial data and concerns of overdosing with the risk of bleeding and anticoagulant-related nephropathy.25 A prudent approach is to check renal function at the initiation of treatment with NOACs, after 3 months and then every year, except for high-risk patients (the elderly >75 years, patients with low body mass) who require monitoring at least every 6 months.26 In patients with declining renal function, the current position of EHRA is to estimate the recheck intervals individually using a simple calculation: if CrCl is ≤60 mL/min, the recheck interval in months is CrCl/10.11
Andreu Cayuelas et al assessed compliance with kidney function monitoring recommendations in patients with non-valvular AF starting NOAC therapy.27 Compliance with kidney function monitoring recommendations was 61%, similar to the group of patients younger than 75 years with a GFR >60 mL/min in this study. Patients younger than 75 years with a GFR >60 mL/min had the highest rate of adequate GFR range, at about 60%, followed by patients ≤75 years old with a GFR <60 mL/min at 29.4% and patients >75 years old at 27.8%. Another noteworthy finding is the low percentage of patients with a GFR range assessed beyond the appropriate 12-month interval in patients >75 years old and in patients ≤75 years old with a GFR <60 mL/min (10.4% and 10.2%, respectively). Family doctors appear to follow an annual pattern for monitoring renal function in all patients receiving NOACs, without individualising the interval for monitoring renal function according to the criteria mentioned here. Therefore, patients who receive annual GFR evaluations have the highest rate of adequate renal monitoring. More training for individualised tracking of patients with other conditions may help Portuguese family doctors improve these results.
There was greater adherence to treatment with edoxaban and less adherence to apixaban, likely due to differences in drug posology, with edoxaban taken once daily and apixaban taken two times per day. Brízido et al evaluated adherence to NOACs and its determinants in a population of patients with AF from the outpatient general cardiology list at a tertiary centre in Portugal. The median adherence was 91% (IQR 74–100%) for rivaroxaban, 87% (IQR 74–100%) for apixaban, 82% (IQR 48–100%) for dabigatran and 96% (IQR 83–100%) for edoxaban. There were no statistically significant differences between the NOACs (p=0.102). Half of the patients (51%) were classified as non-compliant, which is consistent with the findings of this study. It was found that in all NOACs, with the exception of dabigatran, adherence was higher in patients diagnosed after the start of the study than in those diagnosed before the start of the study who had a longer duration of therapy. Therefore, there appears to be greater adherence in the immediate period after the diagnosis than in a later period. Therapy duration, NOACs taken two times per day and higher out-of-pocket costs were independent predictors of non-compliance.12
In another real-world analysis of adherence to NOACs, rivaroxaban and apixaban had favourable profiles compared with dabigatran, and rivaroxaban appeared to have higher overall adherence among the NOACs, although edoxaban was not included in this analysis.28 A systematic review and meta-analysis of observational studies found that up to 30% of patients with AF are non-adherent to NOAC therapy.29 Although we analysed data on drug dispensing in pharmacies, there may be the possibility of patients forgetting to take medication, which is more likely to occur with drugs taken two times per day (apixaban and dabigatran), so medication usage by the patients was not verified.
Limitations
The primary limitation of the AF-React Study is that it relies on AF assessment data coded during the clinical process in primary healthcare. While there are some defects in the coding, it appears that the ICPC-2 K78 encoding has become more common over the years, and since 2015, there has been a decrease in the number of AF diagnoses coded. This suggests that the increase in K78 encoding is related to real new diagnoses rather than simply detecting coding errors in previous diagnoses.
When assessing the appropriateness of the prescribed NOAC dose, only the most recent GFR, weight and age criteria were considered. Unfortunately, other relevant NOAC dosage criteria were not available in the database, namely, the concomitant use of other drugs. It is also important to consider frailty when assessing the appropriate range of GFR to monitor renal function and prescribe NOACs. However, these data were not available for analysis.
Conclusions
The AF-React Study enabled an analysis of the appropriateness of NOAC dosage prescriptions, the appropriate range of GFR for monitoring renal function and prescribing NOACs, and adherence to NOAC intake in patients with AF. As such, the study provides highly relevant conclusions for Portugal. In the future, it will be necessary to improve the appropriate prescribing of NOAC doses and understand the reasons for inadequate monitoring of renal function in patients with AF receiving NOACs. Additionally, further studies are needed to identify reasons for non-adherence to NOAC treatment in patients with AF.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Ethics approval
The Department of Studies and Planning of the Regional Health Administration of Northern Portugal (Ministry of Health, Portugal) processed the data. Anonymised data processing and editing were conducted on a secure platform, and the data were extracted from the server in compliance with legal regulations and with the approval of the Health Ethics Committee of the Regional Health Administration of Northern Portugal (no: 133/2018).
Acknowledgments
Special thanks to all primary healthcare professionals who, in their daily work in the Portuguese health system, fulfil the clinical records and allow researchers like us to obtain evidence that will help us improve the Portuguese’s health.
References
Footnotes
X @mgfamiliarnet
Contributors SSP and CM contributed to the conception of the work. All authors contributed to the design of the study. HM contributed to the data acquisition process. SSP, ASCT and TSH contributed to the analysis and interpretation of the data. SSP drafted the manuscript, while HM, ASCT, TSH and CM critically revised the manuscript. SSP was responsible for the overall content as the guarantor.
All authors gave final approval and agreed to be accountable for all aspects of the work, ensuring integrity and accuracy.
Funding This article was supported by national funds through FCT (Fundação para a Ciência e a Tecnologia, I.P.) within CINTESIS, R&D Unit (reference UIDB/4255/2020).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.