Article Text
Abstract
Introduction The increasing burden of non-communicable diseases, such as hypertension, diabetes and dyslipidaemia, presents key challenges to achieving optimal HIV care outcomes among ageing people living with HIV. These diseases are often comorbid and are exacerbated by psychosocial and structural inequities. This interaction among multiple health conditions and social factors is referred to as a syndemic. In the USA, there are substantial disparities by social position (ie, racial, ethnic and socioeconomic status) in the prevalence and/or control of non-communicable diseases and HIV. Intersecting stigmas, such as racism, classism and homophobia, may drive these health disparities by contributing to healthcare avoidance and by contributing to a psychosocial syndemic (stress, depression, violence victimisation and substance use), reducing success along the HIV and non-communicable disease continua of care. Our hypothesis is that marginalised populations experience disparities in non-communicable disease incidence, prevalence and control, mediated by intersectional stigma and the psychosocial syndemic.
Methods and analysis Collecting data over a 4 year period, we will recruit sexual minority men (planned n=1800) enrolled in the MACS/WIHS Combined Cohort Study, a long-standing mixed-serostatus observational cohort in the USA, to investigate the following specific aims: (1) assess relationships between social position, intersectional stigma and the psychosocial syndemic among middle-aged and ageing sexual minority men, (2) assess relationships between social position and non-communicable disease incidence and prevalence and (3) assess relationships between social position and HIV and non-communicable disease continua of care outcomes, mediated by intersectional stigma and the psychosocial syndemic. Analyses will be conducted using generalised structural equation models using a cross-lagged panel model design.
Ethics and dissemination This protocol is approved as a single-IRB study (Advarra Institutional Review Board: Protocol 00068335). We will disseminate results via peer-reviewed academic journals, scientific conferences, a dedicated website, site community advisory boards and forums hosted at participating sites.
- Hypertension
- HIV & AIDS
- Diabetes & endocrinology
- PUBLIC HEALTH
- EPIDEMIOLOGIC STUDIES
- Sexual and Gender Minorities
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Hypertension
- HIV & AIDS
- Diabetes & endocrinology
- PUBLIC HEALTH
- EPIDEMIOLOGIC STUDIES
- Sexual and Gender Minorities
Strengths and limitations of this study
The largest and most long-standing observational mixed-serostatus cohort of sexual minority men (SMM) in the USA, the MACS/WIHS Combined Cohort Study (MWCCS) provides a large sample of ageing SMM living with diagnostically validated non-communicable diseases.
Because of its legacy of community-based recruitment strategies, the MWCCS minimises the potential selection bias and limited variance that a clinic-based cohort sample could confer on continua of care outcomes.
The MWCCS is not nationally representative, but it is diverse: 44% of the anticipated sample will be racial/ethnic minorities, representing cities in the South, the Midwest, the Rust Belt, the West and the Mid-Atlantic.
Since physiological stress cannot be measured per se, surrogate measures include cortisol levels and heart rate variability. Cortisol can be reliably assayed in hair, though given male baldness some participants will be unable to donate sufficient scalp vertex hair (2–3 cm) for testing.
Background
Over the next decade, more than 70% of people living with HIV (PLHIV) will be older than 50.1 The increasing burden of the non-communicable diseases (NCDs), such as hypertension, diabetes and dyslipidaemia, has already begun to present key challenges to effective HIV care among ageing PLHIV,2–4 the majority of whom in the USA are sexual minority men (SMM).3 5 Studies estimate that by 2030, over 80% of PLHIV will have at least one NCD and almost one-third will have three or more NCDs, leading to potential HIV treatment challenges due to drug–drug interactions and contraindications from co-medications.5
Cardiometabolic NCDs frequently present as comorbid conditions, interact adversely and are inflected by social and structural inequities, forming what is referred to as a syndemic (an NCD syndemic).6–10 Profound and persistent disparities by social position (eg, by race, ethnicity and socioeconomic class) in incidence, prevalence and control of HIV and NCDs exist in the USA,11–16 contributing to disparities in HIV and heart disease mortality rates.16 17
The HIV continuum of care (CoC) framework provides an adaptable heuristic to pinpoint health disparities and care implementation gaps for addressing common NCDs. Designed to identify targets within the HIV care cascade where interventions are most needed,18 19 the HIV CoC delineates proportions of PLHIV in the USA who are undiagnosed with HIV infection (14%), not linked to HIV care (36%), not retained in HIV care (51%) and not virally suppressed (47%).20 Recent research adapting the HIV CoC for diabetes outcomes estimates that in the USA, 28% are unaware of their diabetes diagnosis, of those diagnosed, 5% are not linked to care, 8% are not retained in care and 36% did not meet individualised targets for haemoglobin A1C (HA1c) control,21 which vary by age and number of comorbidities. Similar research on the dyslipidaemia CoC estimates that 30% of US adults are unaware of their high cholesterol and that 51% are not receiving cholesterol-lowering medication.22 Research along the hypertension CoC estimates that 22% of US adults with high blood pressure are unaware of their condition, 27% are not receiving treatment and 50% have uncontrolled hypertension.23 However, NCD continua of care success rates specific to PLHIV have rarely been estimated.
Among SMM, the psychosocial syndemic is a key predictor of HIV CoC disparities. Merrill Singer’s ethnographic research conceptualises that the parallel epidemics of substance use, intimate partner violence (IPV) and HIV, concentrated in impoverished communities, interact synergistically to amplify the burden of disease.7 24 25 Others have built on this work to demonstrate that, among SMM, sexual minority stressors generate a psychosocial syndemic (including stress, depression, substance use and IPV).26–29 Furthermore, the psychosocial syndemic has been shown to be predictive of HIV risk behaviour and incidence,30–32 and has shown to be associated with poor HIV CoC outcomes.33–36
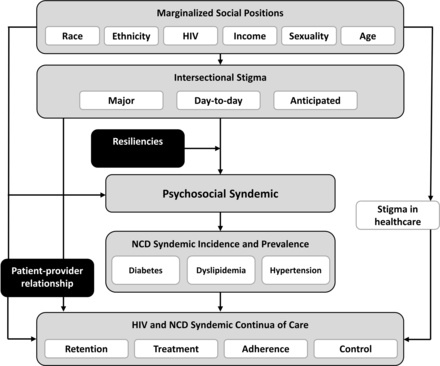
Intersectional stigma may contribute to the psychosocial syndemic and to reduced success along the HIV and NCD continua of care. Intersectional stigma research posits that multiple social and structural stigmas (eg, racism, homophobia and ageism) may increase risk for health inequities in multiply marginalised populations.37–39 Emergent work demonstrates that intersectional stigma is strongly associated with the psychosocial syndemic among SMM of colour.40–42 SMM are frequently subject to multiple, intersecting stigmas that reflect their affiliation with more than one stigmatised group. By causing multiply marginalised populations to avoid situations where they perceive that stigmatisation occurs, such as healthcare environments, intersectional stigma has been associated with suboptimal HIV CoC outcomes, promoting healthcare avoidance and antiretroviral therapy (ART) non-adherence.43 44 Our conceptual model (figure 1) illustrates how the incidence, prevalence and control of diabetes, dyslipidaemia and hypertension might also be formulated within an intersectional stigma framework. Experiencing intersectional stigma may potentiate stress-associated physiological responses that promote the emergence and progression of an NCD syndemic. Stress, particularly stressors related to sexual minority status,45 has been characterised as a key component of the psychosocial syndemic in SMM.26 Physiological stress responses can include lower heart rate variability (HRV), which has been correlated with chronic stress and anxiety46, and elevated cortisol secretion, a reliable biomarker of chronic stress47 48 implicated in the pathogenesis and progression of diabetes, hypertension and dyslipidaemia.49 50
Conceptual framework. NCD, non-communicable disease.
Few studies have prospectively assessed the contribution of intersectional stigma to social disparities in NCD incidence, prevalence and control among PLHIV, and few if any studies have rigorously assessed how the psychosocial syndemic may mediate pathways between marginalised social position and HIV and NCD CoC outcomes. We will build on recent work quantifying intersectional stigma51 52 and the NCD CoC21–23 to assess pathways between social position, intersectional stigma, the psychosocial syndemic and NCD outcomes.
Objectives
The study has three primary aims:
Prospectively assess relationships between social position, anticipated and experienced intersectional stigma and the psychosocial syndemic among middle-aged and ageing SMM living with and without HIV. We hypothesise that (1) multiply marginalised social positions are associated with both higher intersectional stigma (both anticipated and experienced) and psychosocial syndemic burdens, and (2) intersectional stigma mediates the relationship between social position and psychosocial syndemic outcomes.
Prospectively assess relationships between social position and NCD syndemic incidence and prevalence. We hypothesise that (1) multiply marginalised social positions are associated with higher rates of incidence and prevalence of NCD syndemic conditions, and (2) intersectional stigma and the psychosocial syndemic act as serial mediators, contributing to NCD syndemic incidence and prevalence disparities by social position.
Prospectively assess relationships between social position and HIV and NCD syndemic continua of care outcomes, mediated by intersectional stigma and the psychosocial syndemic. We hypothesise that (1) multiply marginalised social positions are associated with poorer outcomes along each step in the NCD and HIV CoCs, (2) intersectional stigma and the psychosocial syndemic act as serial mediators of these pathways and (3) protective factors, including patient–provider trust, resilience, mindfulness and social support, moderate these paths.
Methods and analysis
Study design
Over a 4 year period (June 2023–August 2027, Y1–Y4), we will invite all SMM from nine participating sites in the MACS/WIHS Combined Cohort Study (MWCCS), a long-standing mixed-serostatus observational cohort, to complete self-administered, annual surveys concurrent with MWCCS study visits.53 We will assess five key domains: (1) social position, (2) intersectional stigma, (3) psychosocial syndemic variables, (4) HIV and NCD CoC outcomes and (5) protective moderating factors. In Y3, we will complement psychosocial syndemic data collection by offering participants HRV and hair cortisol concentration (HCC) testing, physiological markers of chronic stress. We will use MWCCS core laboratory and clinical data to estimate incidence and prevalence of NCDs and successful control of NCDs and HIV.
Setting
Rutgers University will act as the main study coordinating centre in collaboration with Georgetown University (survey participant tracking) and the MWCCS Data Analysis and Coordination Centre at Johns Hopkins University (data integration, data set and codebook building). Cohort sites include University of Pittsburgh, Johns Hopkins University, Whitman-Walker Institute, University of California-Los Angeles, Northwestern University, Hektoen Institute for Medical Research, University of Miami, University of Mississippi Medical Centre and University of Alabama-Birmingham.
Participants
We anticipate recruiting 1800 SMM from participating sites. All MWCCS participants must be 18 years of age or older to enrol.
Patient and public involvement
This protocol was reviewed and approved by the MACS/WIHS Combined Cohort Study National Community Advisory Board (MWCCS NCAB). The study instrument was reviewed, pilot-tested and approved by the MWCCS NCAB and the Pitt Men’s Study Community Advisory Board.
Variables and data sources and measurement
Predictors, mediators and moderators
Social position
We will assess social position at each time point, beginning in the year prior to survey administration (Y0). Race, ethnicity and age are collected at baseline via MWCCS core data. Race and ethnicity will be treated as fixed predictors, while older age (≥65) and low-income status will be treated as time-varying predictors. HIV serostatus is assessed at each study visit via ELISA for HIV-seronegative individuals and confirmatory Western Blot to confirm seroconversion, and HIV-1 RNA quantification for PLHIV. Sexual behaviour (eg, sex with men only, sex with men and women) and sexual identity are time-varying, and are assessed annually at MWCCS core visits.54
Intersectional stigma
We will assess intersectional stigma (Y1–Y4) via the Intersectional Discrimination Index (InDI), which uses an intercategorical approach to measure enacted and anticipated stigma.51 We will complement this measure with an intracategorical measure centred on respondent-identified attributions and sources for experienced intersectional stigma in healthcare settings.55
Psychosocial syndemic
To assess current stress, we will use the Perceived Stress Scale (PSS-4).56 We will measure chronic stress using the Trier Inventory for Chronic Stress scale (TICS-E-9).57 To assess physiologic stress-associated biomarkers, we will measure HRV and HCC. Polydrug use will be measured using MWCCS core data assessing frequency of past-year use of each of the following substances: cocaine, crack cocaine, heroin, 3,4-methylenedioxymethamphetamine (MDMA)/ecstasy, gamma hydroxybutyrate (GHB), non-prescription depressants, including opioids, non-prescription stimulants and ‘other street or club drugs’.54 IPV in the past year will be measured using instruments adapted from the RADAR cohort study.58 Depression symptoms will be measured using the shortened Centers for Epidemiological Studies-Depression scale (CES-D-10).59 60 Anxiety will be assessed using the Generalised Anxiety Disorder 2-item screening scale (GAD-2).61
Moderators
Social support will be assessed via the Medical Outcomes Study Social Support brief scale62, resilient coping, with the Brief Resilient Coping Scale63 and patient–provider trust via the Healthcare Relationship Trust Scale.64 Mindfulness will be assessed using the Mindful Attention Awareness Scale.65
Participant satisfaction
Satisfaction with the length and breadth of the questionnaire will be assessed with a one-item question used in prior MACS substudies.66 Additionally, survey respondents will be given open-text space to give feedback on concepts related to stigma and health that they would like future research to explore. Information from these responses will be used to inform potential changes to the survey format.
Survey items were created in English and translated into Spanish for participants who are primarily Spanish-speaking.
Outcomes
NCD syndemic
Consistent with prior research on prevalence of NCD conditions in this cohort,3 67 we will classify participants as having prevalent hypertension if they have had systolic pressure>130 mm Hg or diastolic pressure>80 mm Hg during onsite physical examination and in at least one prior visit and/or prior clinical diagnosis or treatment, as having prevalent dyslipidaemia if they have had fasting total cholesterol≥200 mg/dL, low-density lipoprotein (LDL)≥130 mg/dL, high-density lipoprotein<40 mg/dL or triglycerides≥150 mg/dL in the current visit and at least one prior visit and/or prior clinical diagnosis or treatment, and as having prevalent diabetes if they have had HA1c≥6.5% or fasting glucose level≥126 mg/dL in the current visit and in at least one prior visit and/or prior clinical diagnosis or treatment.3 Participants will be considered to have incident diabetes, dyslipidaemia and/or hypertension if they have no prior history of these respective conditions and have observed values consistent with the ranges above for ≥1 new visits or were newly diagnosed by a healthcare provider or treated for these conditions. These classifications will also constitute denominators for evaluating success along each respective NCD continua of care.
HIV and NCD syndemic continua of care
We will construct care continua for HIV and each respective NCD using historical MACS and prospective MWCCS data. Each MWCCS participant has been screened at each visit since enrolment for HIV, HIV viral load (if living with HIV), hypertension, dyslipidaemia and diabetes and has reported specific use of ART and NCD medications at each visit. As initial care linkage for HIV infection and NCD has likely occurred for most participants with prevalent HIV and NCDs, we will focus CoC measures on gaps in retention in care, treatment for HIV and NCDs, adherence to medications used for treatment and viral suppression and NCD control. To assess retention in care, we will survey participants prospectively using a series of healthcare utilisation questions modified from the National Health and Nutrition Examination Survey.68 For hypertension and dyslipidaemia, participants will be considered as retained in care if they report ≥1 past-year visits corresponding to each of these conditions. For HIV and diabetes outcomes, participants will be considered as retained in care if they report ≥2 past-year corresponding visits.21 69 To assess treatment for HIV and NCDs, we will use MWCCS core data on self-reported medications. These reports are tabulated at each visit and queried for HIV, diabetes, dyslipidaemia and hypertension.70 71 Participants who meet diagnostic definitions for HIV and each NCD and who report no past-year medication use for these respective morbidities will be classified as untreated. MWCCS measures for adherence include annual responses to medication adherence for HIV,72 and for diabetes, dyslipidaemia and hypertension medications in the prior 5 days. To broadly complement these measures, we will assess healthcare avoidance using a one-item measure that has been associated with intersectional stigma in the MWCCS.73
Uncontrolled HIV and NCD syndemic
We will use more conservative clinical and laboratory values to define uncontrolled NCDs than the values we have used to demark participants as experiencing existing NCDs. We define uncontrolled hypertension as blood pressure≥140/90 mm Hg during onsite physical examination, corresponding to stage 2 hypertension and national recommendations for blood pressure-lowering medications.74 We define uncontrolled dyslipidaemia as LDL≥190 mg/dL.74 To assess uncontrolled type 2 diabetes, we will use individualised HA1c control targets consistent with national recommendations.21 75 MWCCS data contain adjudicated outcomes for prior medical diagnoses: for participants without complications (retinopathy, nephropathy or CVD), we will consider HA1c>6.5% as prevalent uncontrolled among those less than 45 years old, HA1c>7.0% as uncontrolled among those 45–64 years old and HA1c>7.5% as uncontrolled among those aged 65 and older. For participants with complications, we consider HA1c>7.0% as uncontrolled for those less than 45 years old, and HA1c>8.0% as uncontrolled for participants≥45 years old. We will define uncontrolled HIV as viral load>20 copies/mL.
Laboratory methods
To assess HRV, we will follow established protocol where mean values are taken from data corresponding to three successive 10 s ECGs using a 12-lead digital recording acquired with General Electric MAC 1200 ECGs in the resting state.76 MWCCS sites will digitally transfer data to the Epidemiological Cardiology Research (EPICARE) Reading Centre at Wake Forest School of Medicine for data cleaning and reading.77 Only ECGs obtained during sinus rhythm and in the absence of arrhythmias, conduction abnormalities and >50% ectopy will be analysed. Heart beats immediately before and after ectopic beats will be excluded from measurement. We will obtain mean values of SDNN (SD of all normal-to-normal R–R intervals) and rMSSD (root mean square of successive differences between normal-to-normal R–R intervals).
To assess HCC, we will follow established and validated protocols. Hair samples from each consenting participant with adequate scalp vertex hair will be collected from participants who consent to providing this sample at each participating site. Samples will be stored and sent in batches to Technische Universität Dresden (TUD). Following published protocols, TUD will convert results of assay readouts to picograms of cortisol/milligram dry hair weight.78–81
Statistical methods
Quantitative analysis and sample size
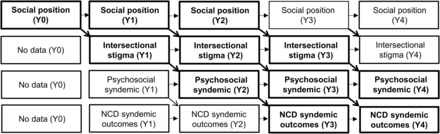
To evaluate aim 1, we will construct a generalised structural equation model (gSEM) using a cross-lagged panel model (CLPM) design,82 beginning with year 0 values for predictors (marginalised social positions, via MWCCS core data from visit preceding this substudy), year 1 values for the mediator (intersectional stigma) and the moderators (resiliencies), year 1 values for moderators (resiliencies) and year 2 values for outcomes (psychosocial syndemic) (see figure 2).
Cross-lagged panel model (CLPM) approach showing paths between social position, intersectional stigma, the psychosocial syndemic and NCD syndemic outcomes. Bold paths show full temporal progressions. NCD, non-communicable disease.
We will create latent variables encompassing each of these domains. This approach will allow us to assess temporal effects of (1) marginalised social position on intersectional stigma burden, (2) intersectional stigma burden on the psychosocial syndemic, (3) marginalised social position on the psychosocial syndemic, mediated by intersectional stigma burden and (4) moderation of the intersectional stigma burden→psychosocial syndemic path by resiliencies. By leveraging year 0 social position data, three full temporal progressions will be available to assess aim 1. Mediation will be measured by assessing total, direct and indirect effects. We will assess moderation of the stigma→psychosocial syndemic path using the latent moderator structural equations method (LMS).83
To achieve aim 2, we will construct a gSEM that treats marginalised social position (except age, which is a confounder) and NCD syndemic prevalence (diabetes, dyslipidaemia and hypertension) as latent variables, in order to assess overall associations between marginalisation burden and prevalent NCD syndemic burden. For each NCD syndemic condition, we will use NCD prevalence data from baseline (Y0) to establish a denominator of at-risk (non-prevalent) SMM for use in NCD incidence analyses. Cases in the at-risk group will be classified as incident if laboratory values are consistent with thresholds for diabetes, dyslipidaemia and hypertension for ≥2 consecutive annual visits.3 To estimate the effects of social position (Y0/Y1) on NCD syndemic incidence (Y3/Y4), we will construct a series of gSEM with the CLPM approach, using a Poisson distribution. We will model incidence rate ratios of pooled NCD syndemic incidence (eg, any new NCD condition) in Y3 and in Y4 as a function of social position. To optimise interpretability of results, we will make use of the social position classifications used in our NCD prevalence models (permutations of ethno-racial and low-income status groupings, treating White, non-Hispanic, non-impoverished SMM as the referent category). To assess serial mediation,84 85 we will then add intersectional stigma (Y1/Y2 values) and—first separately, and then conjointly—each psychosocial syndemic variable (Y2/Y3) to these models. We will use modelled data to estimate total, direct and indirect effects between social position, intersectional stigma, the psychosocial syndemic and NCD syndemic incidence. Based on prior research demonstrating potential for confounding on either main effects or mediators, covariates will include bisexuality, older age, HIV status, body-mass index, lifetime cigarette use (pack-years),86 physical activity87 and cumulative lifetime ART use.88
To assess aim 3, we will first provide descriptive statistics showing failure rates across retention, treatment, adherence and control domains of the HIV CoC and each respective NCD CoC (diabetes, dyslipidaemia and hypertension), using prevalent cases by Y1 to establish respective denominators for each CoC. We will next construct a series of cross-sectional (Y1) gSEM to model the overall effect of marginalised social position on the odds of failure across each respective CoC outcome. We will add intersectional stigma and each psychosocial syndemic variable to this model, testing for serial mediation by assessing total, direct and indirect effects.84 85 Finally, to evaluate the prospective effects of stigma on the NCD syndemic CoC, we will construct a series of CLPM in gSEM, stratified by HIV status. We will model direct, indirect and total effects between the predictor (Y0/Y1 social position), the first mediator (Y1/Y2 intersectional stigma), the second mediator (Y2/Y3 psychosocial syndemic) and each syndemic NCD/HIV CoC outcome (Y3/Y4). Using the LMS method, we will assess moderation of the psychosocial syndemic→NCD/HIV CoC outcomes path by patient-provider relationship strength. We will conduct parallel analyses with the PLHIV subsample in order to assess specific HIV CoC outcomes. Covariates include lifetime cigarette use,86 body-mass index, physical activity87 and cumulative ART use.88 By leveraging Y0 social position data, two full temporal progressions will be available to assess aim 3. All gSEM model fits will be assessed using the Yuan-Bentler goodness-of-fit test, which analyses covariance structures by fitting sequential single-level models.89
The anticipated overall sample size in the study is estimated to be n=1800 (PLHIV n=988), corresponding to 7200 person-observations over four consecutive annual visits. Methods for estimating sample size for gSEM with latent variables have not achieved scientific consensus. Long-standing practice has suggested an overall minimum n=200,90 and more recent work suggests a range of n=30–460,91 with this range dependent on missing data, number of latent factors and strength of factor loading.91 MacKinnon has posited that an n=500 confers sufficient power (power=0.8, α=0.05), to detect small mediation effects using a cross-sectional study design.92 Prior research from this cohort has shown strong effects of adulthood discrimination on NCD prevalence,73 indicating that a more specific and robust stigma instruments deployed longitudinally will likely be sufficiently powered to show significant associations with key outcomes.
Recruitment
The study team staff at each site will contact eligible MWCCS participants and invite them to participate in this study.
Ethics and dissemination
Research ethics
Per NIH guidelines for multisite research, the study utilises a single IRB (sIRB), where the Advarra Institutional Review Board (IRB) serves as the sIRB of record for all participating sites. Given minimal risk to participants, all data and safety monitoring will be conducted by the Principal Investigator, who will provide oversight of data collection and management processes and ensure proper communication with the sIRB as need arises.
Consent
Study staff at each participating site will obtain consent from eligible participants during their respective MWCCS visits. Staff who obtain consent must have current human subjects research certifications via CITI and experience obtaining consent for the MWCCS.
Consent will take place in-person or via phone prior to performing any research procedures. For subjects who are consented via phone, Docusign will be used to capture electronic written signature. Potential subjects will be informed that participation is voluntary, their decision whether to participate will not affect their care or relationship with each respective participating site and they may withdraw their consent and authorisation at any time.
Dissemination plan
We will present study findings in peer-reviewed academic journals and at professional conferences. Strategies will include sharing results annually at local and National Community Advisory Board meetings, and posting findings on a study-specific website, which will provide portals to published papers, recorded video presentations by investigators geared towards community dissemination and links to measures and concept sheet submissions forms for interested external collaborators.
We will elicit participant feedback from participants by hosting community forums each site at the end of the final year of data collection, using an approach with basis in World Café methodology,93 which study investigators have used in partnership with diverse populations to develop evidence-based HIV CoC interventions.94–98 These forums are intended to share overall results, generate discussions with SMM about interpretation and salience of findings, and facilitate collective dialogue about potential intervention frameworks. These discussions will highlight resiliencies indicated by study data to promote successful HIV and NCD control in the face of social marginalisation and consequent intersectional stigma, pinpoint data-supported gaps along the HIV and NCD syndemic CoC that participants view as amenable to intervention and brainstorm relevant strategies and settings that SMM view as promising for intervention development. As few intersectional stigma reduction interventions exist,99 there is a need to delineate targets and generate concepts for intervention design.100 We anticipate this collective dialogue to result in one or more promising intervention concepts that can be tested in the future using a trial design.
Data deposition and curation
Data will be collected through a combination of surveys, core MWCCS data integration, HCC collection and HRV collection, as previously described (see table 1). Survey data will be collected electronically via Qualtrics using confidential links and stored on Rutgers University servers, every 6 months, survey data will be transferred to the MWCCS Data Analysis and Coordination Centre at Johns Hopkins University using a HIPAA-compliant Sharepoint link. MWCCS participant identifiers will be used by each participant to access surveys. Consent forms will be stored according to security protocols at each participating site. Electronic ECG data from each study site will be securely transferred to EPICARE for analysis using a secure file transfer protocol service. Hair cortisol samples will be sent to TUD using 10-digit MWCCS identifiers on individually bagged samples. ECG and HCC data will then be transferred using two-way encrypted file sharing services to Johns Hopkins University (JHU) for core data integration, survey data will be transferred to JHU using the Rutgers HIPAA-compliant Box service for core data integration. JHU will make integrated, deidentified data available to the investigative team via HIPAA-compliant Box. Internal and external researchers interested in using these and other MWCCS-associated data can access these data by submitting concept sheets via the parent study website (www.mwccs.org).
Study measures
Supplemental material
Ethics statements
Patient consent for publication
Acknowledgments
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos, David Hanna and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Centre (Gypsyamber D’Souza, Stephen Gange and Elizabeth Topper), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora and Michelle Floris-Moore), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung and Blood Institute (NHLBI), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute On Aging (NIA), National Institute of Dental and Craniofacial Research (NIDCR), National Institute of Allergy And Infectious Diseases (NIAID), National Institute of Neurological Disorders And Stroke (NINDS), National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), National Institute of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD) and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), P30-MH-116867 (Miami CHARM), UL1-TR001409 (DC CTSA), KL2-TR001432 (DC CTSA) and TL1-TR001431 (DC CTSA). The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @KathyWuMD
Contributors The study concept and design was conceived by MRF. MWP, ACT, DLJ, DK-P, SAH, JK, JM-T, KW and LB assisted in refining the study questionnaires and study design. SB, SAH, JM-T, JK, DK-P, DLJ, MJM, VS and M-CK are responsible for data collection. Analyses will be conducted by MRF, ACT, DW, SAH and MWP. MRF prepared the first draft of the manuscript. All authors critically revised the manuscript and approved the submitted version.
Funding Funding for this study is being provided by the US National Institutes of Health, National Heart, Lung and Blood Institute (7R01HL160326; PI: MRF). The funder had no role in the design of the study, data collection, data analyses, interpretation of data or preparation of this manuscript.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.