Article Text
Abstract
Introduction The subtransverse process interligamentary (STIL) plane block is an emerging interfascial plane block that has garnered attention for its potential to provide effective postoperative analgesia for breast and thoracic surgeries. However, a direct comparative assessment between the STIL plane block and the paravertebral block is currently lacking. Consequently, our study aims to assess the analgesic efficacy of the STIL block in comparison to paravertebral block for patients undergoing video-assisted thoracoscopic surgery (VATS).
Methods and analysis This study is a randomised, parallel-controlled, double-blind, non-inferiority trial, with the goal of enrolling 114 participants scheduled for uniportal VATS at Shanghai Pulmonary Hospital. Participants will be randomly assigned in a 1:1 ratio through block randomisation to receive either the STIL plane block (n=57) or the paravertebral block (n=57). The primary outcome of the study is the area under the curve of Numerical Rating Scale(NRS) scores recorded over a 48-hour period following the surgical procedure. Secondary outcomes encompass the evaluation of Quality of Recovery-40, cumulative sufentanil consumption, serum inflammatory factors, rescue medication usage, the incidence of adverse events and the patient satisfaction scores.
Ethics and dissemination This study has received approval from the Medical Ethics Committee of Shanghai Pulmonary Hospital (approval no. L22-329). Written informed consent will be obtained from all participants. The findings will be submitted for publication in peer-reviewed journals.
Trial registration number ChiCTR2200066909.
- Patients
- Adult anaesthesia
- Pain management
- Cardiothoracic surgery
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study aims to assess the safety and efficacy of the subtransverse process interligamentary plane block as a perioperative analgesia method for patients undergoing video-assisted thoracoscopic surgery.
The study is a parallel, non-inferiority, randomised controlled trial with patient and assessor blinding.
The study focuses on patient-reported outcomes during the early postoperative period.
Limitations of the study include the absence of investigation into long-term effects, lack of sensory plane testing and constraints related to drug dosage.
Introduction
Surgical advancements and the use of video-assisted thoracoscopic surgery (VATS) have gained popularity in lung cancer management.1 Addressing postoperative pain in thoracoscopic patients has been a hot topic of research. Uniportal VATS, a minimally invasive technique using a single incision, offers benefits including diminished postoperative pain, shorter hospital stays and enhanced cosmetic outcomes compared with traditional multiport VATS.2 However, acute postoperative pain remains a prevalent symptom following uniportal VATS.3 4 Inadequate pain management can negatively affect recovery, increase the risk of pulmonary complications and contribute to the development of chronic postsurgical pain.5 Therefore, the development of perioperative analgesic techniques and strategies for VATS patients holds paramount significance.
Thoracic paravertebral block (TPVB) is a common method for managing postoperative pain after thoracoscopic surgery.5 It provides analgesic effects akin to those of thoracic epidural block and is the preferred regional anaesthesia technique for thoracoscopic surgery.6 However, TPVB is technically challenging, requiring skilled healthcare professionals.7 The narrow paravertebral space, located between the superior costotransverse ligament (SCTL) and the parietal pleura, poses a risk of inadvertent pleural puncture and vascular damage, and increased the potential for pneumothorax and haematoma.7 8
The subtransverse process interligamentary (STIL) plane block is a recently introduced technique that holds promise as an alternative to TPVB.9 STIL plane block specifically targets the region adjacent to the paravertebral space, rather than directly penetrating it, which theoretically diminishes the potential risks of inadvertent pneumothorax and hematoma compared with TPVB.9 Additionally, due to its close anatomical proximity to the paravertebral space, the STIL plane block may facilitate a more straightforward dispersion of local anaesthetics into this area.10 Research has also confirmed that the block achieved with STIL plane block is effective in providing adequate pain relief for breast surgeries.11 These findings suggest that STIL plane block holds promise as a viable alternative to TPVB in patients undergoing thoracic surgery. There is a paucity of clinical trials comparing the differences between the STIL plane block and TPVB for perioperative analgesia. Therefore, we hypothesise that the STIL plane block will demonstrate non-inferiority to TPVB in terms of postoperative analgesia for patients undergoing VATS.
Methods and analysis
Study design and setting
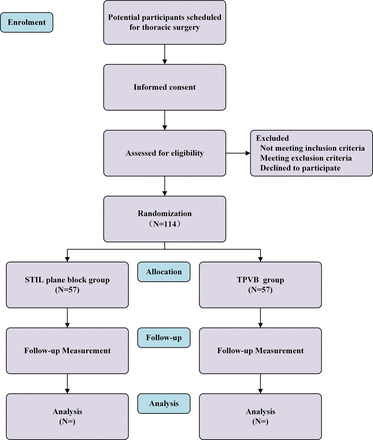
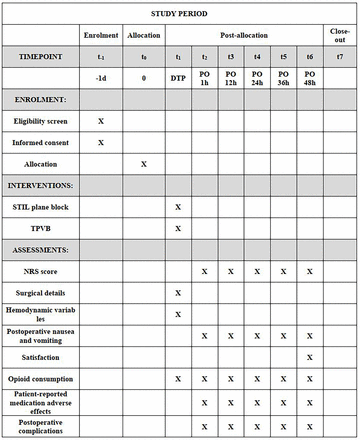
This study is a randomised, parallel-group, non-inferiority trial being conducted at Shanghai Pulmonary Hospital. The study is scheduled to commence in January 2023 and conclude in December 2027. The study design adheres to the guidelines outlined in the Standard Protocol Items for Randomised Trials (SPIRIT). Figure 1 presents the Consolidated Standards of Reporting Trials flow chart, and figure 2 includes the SPIRIT figure, with an accompanying checklist available as online supplemental file 1.
Supplemental material
CONSORT flow diagram for the study. CONSORT, Consolidated Standards of Reporting Trials; STIL, subtransverse process interligamentary; TPVB, thoracic paravertebral block.
Schedule of enrollment, interventions and assessments following the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) guidelines. DTP, during the procedure; NRS, Numerical Rating Score; PO, postoperative; QoR-40 score, Quality of Recovery-40; STIL, subtransverse process interligamentary; TPVB, thoracic paravertebral block.
Participants
Inclusion criteria
Age: 18–64 years.
American Society of Anesthesiologists physical status classifications I–II.
Exclusion criteria
History of prior chest surgery, morbid obesity (body mass index >40 kg/m2),12 severe cardiovascular system diseases, chronic respiratory system diseases (chronic obstructive pulmonary disease, asthma, interstitial lung diseases and idiopathic pulmonary fibrosis), liver or kidney dysfunction, blood system disorders or a history of mental illnesses.
Known allergy to local anaesthetics.
Presence of local infection at the block site or systemic infection.
Language barriers or communication difficulties.
Patient refusal to participate in the study or unwillingness to use the analgesic pump.
Recruitment
All patients scheduled for VATS will undergo eligibility screening 1 day before the scheduled operation. Eligible patients will be given the opportunity to enrol in our study, during which we will provide them with detailed information about our research. Each patient will receive comprehensive information about their role in our study and will be assured that their personal information will be kept strictly confidential.
Informed consent
Prior to enrolling in our study, informed consent will be obtained from each patient or their legally authorised representative (LAR), as detailed in online supplemental file 2. This consent process will emphasise that participation is entirely voluntary, and participants have the right to withdraw from the study at any point without affecting their access to medical care. No study procedures will commence until informed consent has been obtained.
Supplemental material
Randomisation and blinding
Following the acquisition of signed informed consent from the patient or their LAR, patients will be randomly allocated to either the STIL plane block group or the TPVB group in a 1:1 ratio. Randomisation will be executed using sealed envelopes, which will be available at Shanghai Pulmonary Hospital. A masked researcher will generate treatment assignments by using a computer-generated random number list with block sizes of 6, as generated by Stata V.16.0 (STATA). The research assistant (RA) will create randomised envelopes just prior to the patient randomisation process and ensure the envelopes’ integrity and presence during each monitoring visit.
The RA, who will be unaware of the randomised patient assignments, will conduct all baseline interviews. Patients will be kept uninformed about their respective interventions, and research staff responsible for completing the postprocedural follow-up questionnaire will also be blinded. While it is not possible to blind anaesthesiologists involved in patient care, the surgical team will be kept unaware of the group assignments.
Standard anaesthetic and analgesic management
On the day of the operation, patients will be admitted to the operating room. Vital signs, including heart rate, blood pressure (BP) measurement, including systolic BP, diastolic BP, pulse pressure and mean arterial pressure, oxygen haemoglobin saturation measured by pulse oximetry, end-tidal carbon dioxide partial pressure (EtCO2) and urine output, will be continuously monitored throughout the surgery. Prior to anaesthesia induction, an intravenous catheter will be placed in the right internal jugular vein under the guidance of ultrasound after local anaesthesia. Preoxygenation with 100% oxygen for 3 min before anaesthesia induction will be administered via a face mask.
Anaesthesia induction will be initiated with midazolam (0.05 mg/kg), propofol (1–2 mg/kg), sufentanil (0.5–0.7 µg/kg) and 0.6 mg/kg rocuronium bromide. Subsequently, double-lumen endobronchial intubation will be performed for mechanical ventilation, with confirmation of the placement of a double-lumen endobronchial tube (DLT) using a fibreoptic bronchoscope. The ventilation strategy employed will adhere to a one-lung protective approach, characterised by tidal volumes of 6 mL/kg or lower, based on predicted body weight, and a positive end-expiratory pressure of 5–10 cmH2O, following established guidelines and expert recommendations.13 The respiratory rate will be adjusted to maintain EtCO2 levels within the range of 35–45 mm Hg. After intubation with a DLT, anaesthesia maintenance will involve the administration of propofol and remifentanil to achieve a spectral entropy value between 40 and 60.
Following extubation in the operating room, patients will be moved to the postanaesthesia care unit. As a preventive measure against postoperative nausea and vomiting (PONV), patients will receive intravenous dexamethasone (5 mg) and tropisetron (5 mg) prior to the initiation of general anaesthesia.
Postoperative pain management will strictly adhere to a standardised protocol for all patients. This protocol encompasses the administration of a combination of 5 µg of sufentanil and a 50 mg intravenous infusion of flurbiprofen axetil administered 30 min before the conclusion of surgery. Additionally, patients will receive a daily intravenous infusion of 50 mg of flurbiprofen axetil for postoperative analgesia. Patient-controlled analgesia (PCA) will be implemented using a 24-hour infusion of sufentanil at a concentration of 1 µg/mL solution. The PCA protocol will consist of an infusion rate of 2 mL/hour, a 2 mL bolus dose, a lockout time of 15 min and a maximum limit of 10 mL/h. PCA treatment will be initiated based on a Numerical Rating Scale (NRS) score >2.14–16 Oxycodone 5 mg/acetaminophen 325 mg will be available as a rescue analgesic. In addressing PONV, a 5 mg dose of intravenous tropisetron will be administered in the hospital ward.
Interventions
Immediately after the induction of general anaesthesia, the blockades will be carried out with patients positioned in a lateral decubitus position. This approach is chosen to mitigate patient anxiety and discomfort, while also ensuring blinding to the intervention allocation.16 17 All blockades will be administered by experienced anaesthesiologists well versed in ultrasound-guided regional blocks.
The TPVB procedure will be conducted through an in-plane transverse approach, following established techniques.18 On achieving an optimal ultrasound image, which includes clear visualisation of the transverse process, a wedge-shaped hypoechoic paravertebral space, and the parietal pleura, the needle will be advanced from a lateral to medial direction until the needle tip penetrates the internal intercostal membrane. The accurate placement of the needle will be confirmed by observing the downward displacement of the pleura during the injection of the local anaesthetic.
The STIL plane block will be administered using an in-plane technique, following the method described by Kilicaslan et al.9 This procedure entails identifying key anatomical landmarks, including the identification of the intertransverse ligament, transverse process, SCTL and pleura through a parasagittal ultrasound scan. Subsequently, the needle will be advanced in-plane in a caudal to cranial direction, with continuous ultrasound guidance employed to ensure precise needle placement.
Patients randomised to receive STIL plane block and TPVB will be provided with a 15 mL mixture of local anaesthetics, comprising a 1:1 mixture of 15 mL of 1% ropivacaine and 2% lidocaine, at the T5–6 intercostal levels.19
Data collection, monitoring and management
Preoperative, intraoperative and postoperative follow-up data will be meticulously extracted from electronic medical records, monitoring devices and pertinent manual records by the research team. This data will be documented on standardised paper forms and later double-entered into Epidata software V.3.1 by two proficient RAs.
The data safety and monitoring board (DSMB) will be constituted of two senior anaesthesiologists and one surgeon, all of whom will maintain a blinded status with regard to the study. The DSMB will provide independent oversight of the trial, conducting a comprehensive review of participant safety and data storage throughout the study.
Outcomes
Primary outcome
The primary outcome measure will be the area under the curve (AUC) of NRS scores for pain experienced during deep inspiration over the initial 48 hours following surgery. Pain assessments will be systematically conducted at the 1, 12, 24, 36 and 48 hours postoperative time points.
Secondary outcomes
AUC of NRS scores for pain at rest over a 48-hour period.
Time to the initial administration of sufentanil analgesia.
Incidence of postoperative opioid-related side effects such as nausea, vomiting and dizziness, as well as other complications.
Patient satisfaction with the effectiveness of analgesia during the initial 48 hours postoperatively, assessed using a five-point Likert scale, ranging from ‘highly unsatisfactory’ to ‘highly satisfactory.’
Quality of Recovery-40 assessment.20 21
Plasma biomarker concentrations ()measured using ELISA both before the operation and on the first day after surgery.
Incidence of adverse events, as detailed in online supplemental file 3.
Supplemental material
Statistical methods
Sample size
Based on data from our previous unpublished study, the mean cumulative pain score, calculated as the AUC from 1 to 48 hours following surgery, was estimated to be 77.6 (14.7) for the TPVB group and 102.1 (19.2) for the STIL group. With a power of 80%, a one-sided significance level of 2.5%, and a non-inferiority limit of 34 for NRS AUC (a 33% difference between treatment groups),22 a minimum sample size of 51 subjects per group was calculated. Considering a potential drop-out rate of 10%, 57 patients per group were enrolled.
Descriptive statistics
Continuous variables will be presented as means and SDs for normally distributed data. For continuous variables with non-normally distributed data, medians and ranges will be reported. Categorical data will be described using counts, proportions and risk ratios with 95% CIs.
Planned outcome analysis
For the primary outcome, both the intention-to-treat and per-protocol (PP) approaches will be used. The normality of the distribution of AUC of NRS scores will be assessed using the Shapiro-Wilk test. Normally distributed variables will be reported as mean±SD and analysed with independent t-tests, while non-normally distributed variables will be reported as median (IQR) and analysed with the Mann-Whitney U test.
For secondary outcomes, the PP approach will exclusively be used. Comparative analysis of secondary endpoints between the two treatment groups will be performed using Student’s t-test (or Mann-Whitney U test if necessary) for continuous quantitative variables and the χ2 test (or Fisher’s exact test) for qualitative variables. Multivariate analyses will encompass linear and logistic models. Time-to-event analyses will use the Kaplan-Meier method and the Cox proportional hazards model.
P values will be two tailed with a significance threshold of 0.05. The statistical analyses will be conducted using Stata V.16.1 (StataCorp), R V.4.0.3 (the R Foundation) and GraphPad Prism V.8.0 (GraphPad Software, San Diego, California, USA).
Patient and public involvement
None.
Ethics and dissemination
Ethics approval and consent to participate
This clinical study will adhere to the principles of the Declaration of Helsinki and will be conducted in strict compliance with the approved protocol, good clinical practice, designated standard operating procedures, and all relevant local laws and regulations applicable in the country where the study is conducted. The study protocol has received ethical approval from the Ethics Committee of Shanghai Pulmonary Hospital, China (approval no. L22-329). Informed consent will be a mandatory requirement for all participating patients.
Dissemination policy
The results of this study will be disseminated without regard to the impact of the intervention on study outcomes. A manuscript detailing the intervention’s effects will be submitted to a peer-reviewed journal on completion of data collection and analysis.
Trial status
Recruitment for this trial commenced in January 2023 and is expected to conclude by December 2027. The protocol version number is V.2.0.
Discussion
Lung cancer represents a global health concern as one of the most fatal malignancies.16 But recent advancements in thoracoscopic surgery have elevated it as a pivotal therapeutic approach.23 Uniportal thoracoscopic surgery, characterised by its smaller incision, has gained significant popularity, promising faster recovery, reduced complications, improved aesthetics and less postoperative pain.24–26 However, postoperative pain remains a common issue for uniportal thoracoscopic surgery patients, significantly hindering their recovery.27
Although thoracic surgery employs a variety of regional blockade techniques, including TPVB, thoracic epidural anaesthesia (TEA), serratus anterior plane (SAP) blocks, and erector spinae plane blocks, retrolaminar block, combined deep and superficial SAP block, serratus posterior superior intercostal plane block,28 29 ongoing debate persists regarding the selection of the most effective method.22–24 The administration of TEA necessitates a high level of technical expertise and is associated with specific adverse effects that may adversely affect postoperative recovery.30 TPVB is a widely used and guideline-recommended approach for pain management.8 31 This technique entails the injection of a local anaesthetic into the thoracic paravertebral space to block the thoracic spinal nerve, its branches and the sympathetic trunk, delivering analgesic effects comparable to epidural blocks. Nevertheless, TPVB carries inherent risks due to the delicate nature of needle insertion, including potential complications such as pneumothorax and haemothorax. Therefore, alternative approaches are continuously being explored.
The STIL plane block, a relatively recent technique introduced by Kilicaslan et al, is believed to pose a lower risk of severe complications compared with paravertebral block.9 11 This lower risk primarily arises from its injection into a tissue plane away from potentially problematic structures. Additionally, it is hypothesised that the STIL plane block can effectively provide pain relief by blocking both dorsal and ventral rami of the spinal nerves.11 Its close anatomical proximity to the paravertebral space may also facilitate a more straightforward dispersion of local anaesthetics, theoretically achieving a similar effect as TPVB.10 11 Taking into account these factors, along with the reduced trauma associated with uniportal VATS,32 33 which avoids rib spreading, we have designed a randomised controlled study to investigate whether the STIL plane block can provide pain relief non-inferior to TPVB in uniportal VATS.
Our study has several limitations. One limitation is that we only assess NRS scores within 48 hours postoperatively. This restricted time frame may not offer a comprehensive understanding of the complete postoperative analgesic effect. Another limitation is the inability to conduct sensory testing due to the nerve blockades administered after the induction of general anaesthesia. Additionally, the use of 15 mL local anaesthetic in our study might impose certain constraints on the study outcomes.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
WW and DW contributed equally.
Contributors WW and HS designed the study. DW, WW and TZ collaborated on manuscript drafting. WH made significant contributions to the study’s conception and design. YL developed the statistical analysis plan and estimated the sample size. HS contributed to study design, critical revisions and final manuscript approval. All authors acknowledge their accountability for all aspects of the work, ensuring its accuracy and integrity.
Funding This work was supported by Shanghai Special Program for Research on Aging and Women and Children's Health (No. 2020YJZX0136) and Talent Program of Shanghai Pulmonary Hospital (No. fkgg1809, fkxr1902).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.