Article Text
Abstract
Introduction Urinary incontinence (UI) is one of the most common chronic diseases among women, which can endanger their physical and mental health and incur a heavy financial burden on both individuals and society. Web-based interventions (WBIs) have been applied to manage women’s UI, but their effectiveness has remained inconclusive. This systematic review and meta-analysis aims to explore the effectiveness of WBIs on self-reported symptom severity, condition-specific quality of life, adherence to pelvic floor muscle training (primary outcomes) and other extensive secondary outcomes among women with UI. We also aimed to investigate whether intervention characteristics (format, interactivity and main technology) have impacts on the effectiveness of primary outcomes.
Methods and analysis This systematic review protocol was developed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols guidelines. 10 electronic databases will be comprehensively searched from their inception to 1 May 2024, along with grey literature searches and manual reviews of relevant reference lists to identify eligible randomised controlled trials. The methodological quality of the included studies will be assessed by two reviewers based on the Cochrane Risk of Bias Tool. Meta-analyses will be conducted via Stata V.12.0. Leave-one-out sensitivity analyses will be performed, and publication bias will be evaluated using funnel plots and Egger’s test. Subgroup analyses regarding intervention format, interactivity and main technology will be carried out.
Ethics and dissemination No ethics approval is needed for this review since no primary data are to be collected. The results of this review will help develop an optimal WBI for women with UI, thereby providing them with maximum benefits. The findings will be disseminated via a peer-reviewed journal or conference presentation.
PROSPERO registration number CRD42023435047.
- eHealth
- Meta-Analysis
- Systematic Review
- Urinary incontinences
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This systematic review protocol strictly adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines to ensure quality in all aspects of study planning, execution and reporting.
We will apply a broad search strategy to search ten electronic databases.
Subgroup analyses regarding intervention format, interactivity and main technology will be conducted if possible to provide scientific evidence for researchers and healthcare professionals to optimise the web-based intervention regimen.
Anticipated high heterogeneity across available studies may increase the difficulty in interpreting a meta-analysis.
Another potential limitation of this systematic review may be the introduction of language bias since the search will be restricted to studies published in English and Chinese.
Introduction
Urinary incontinence (UI) is defined by the International Urogynecological Association and the International Continence Society as ‘complaints of any involuntary leakage of urine’.1 It is one of the common chronic diseases that endanger women’s health, affecting at least 200 million women around the world.2 Estimates of the prevalence of UI are contested and vary widely (from 5% to 70% globally) depending on the definition applied, population investigated and measurement tools used,3 with most studies reporting a prevalence of any UI in the range of 25%–45%.4 Worse still, this prevalence is expected to be higher among women who have risk factors such as being overweight or obese and having a higher parity, and estimates rise with each decade of life as a consequence of the ageing population.5 In short, UI has become a key public health and social problem around the world.
Although UI is not life-threatening, it can be a debilitating condition that significantly impacts the quality of life (QoL) in both physical and psychological aspects for the majority of women affected. Evidence reveals that UI interferes with women’s daily lives, including work, household duties, recreational life, and even sleep, and makes them suffer from psychological distress (such as embarrassment, low self-esteem and depression).6 It is also closely associated with numerous severe medical conditions (eg, urinary tract infections, perineal dermatitis and pressure wounds).7 In the medium or long term, UI in older women significantly increases the risk of falls and being referred to nursing homes.7 Furthermore, women with UI often find themselves socially isolated and relatively inactive,6 leading to an increased risk of suicide.8 From the perspective of economics, UI imposes a considerable financial burden on both individuals and the healthcare system. For example, the USA allocates approximately US$12 billion annually to cover expenses related to therapeutic management, absenteeism and disability associated with UI,9 and in the UK, the expenditure exclusively on UI containment products, such as absorbent pads, amounts to approximately £80 million per year.10 Considering the substantial adverse consequences that UI may cause, it is crucial to take measures to manage UI effectively.
Existing evidence-based UI treatments can be broadly separated into surgeries, pharmaceutical therapies and conservative pelvic floor rehabilitation treatments (hereinafter referred to as conservative treatments).11 Incontinence surgeries and pharmaceutical therapies often exhibit only modest effectiveness but commonly lead to several side effects.12 In contrast, conservative treatments, which mainly include pelvic floor muscle training (PFMT), lifestyle intervention (such as weight loss, smoking cessation and fluid intake management), bladder training and electrical stimulation, are considered to be relatively low-risk, cheaper and can be initiated by most women without extensive preliminary evaluation12; these advantages make them seem more attractive in UI treatments than surgeries and pharmaceutical therapies.13 Substantial evidence has demonstrated that conservative treatments can cure or ameliorate symptoms in about two-thirds of patients with UI,14 15 thereby reducing disease burden and optimising health outcomes. It is particularly noteworthy that PFMT has been recommended by the International Continence Society as the first-line treatment for UI since 2005.14 However, despite the existence of several effective UI treatments, the uptake of professional treatments is poor. It is reported that only a small percentage (around 25%–30%) of women with UI seek professional help,16 which could be attributed to the fact that most UI treatments are currently typically administered through ‘face-to-face’ sessions by professional physiotherapists or registered nurses in clinics or hospitals,12 with the stigma of UI, inconvenient traffic, time constraints and high medical costs constituting the main obstacles to their uptake.17–19 Meanwhile, most healthcare settings do not routinely provide treatments and management practices for patients with UI due to limited human and material resources.20 Given the multiple deficiencies of the traditional mode of UI management, it is imperative to identify a more feasible, expandable, sustainable, affordable and privacy-friendly mode of care to manage UI among women effectively and simultaneously alleviate the healthcare system’s burden without affecting its current services or compromising care quality.
Web-based interventions (WBIs), referring to achieving specific health objectives via web-connected devices such as smartphones, computers,and laptops,17 21 might be an effective complement and alternative to narrow the aforementioned gaps and manage women’s UI more effectively through an innovative delivery mode. The benefits of interventions that are delivered via the web include anonymity, relatively low cost and convenience because they enable individuals to receive interventions at anytime and anywhere without face-to-face contact with professionals,22–24 thereby reducing stigma, transportation costs and waiting time for treatments, which are particularly suitable for those with busy schedules or who require flexibility owing to work and family responsibilities. Recent systematic reviews have demonstrated that WBIs can enhance health outcomes among patients with chronic diseases such as diabetes25 and dementia.26
Moreover, with the rapid growth of internet access rates worldwide and the popularisation of web-based devices, the practical feasibility of WBIs has increased.17 25 26 In recent years, the increasing number of randomised controlled trials (RCTs)27–31 related to WBIs on women’s UI, in particular in the context of the COVID-19 pandemic, has demonstrated a growing need for supplementary strategies that can enhance existing services and provide better assistance to UI management for women. Nevertheless, the findings about the effects of WBIs on ameliorating health outcomes for UI women are not consistent. For instance, some trials reported that, compared with the control group, WBIs significantly improved self-reported symptom severity,28 30 condition-specific QoL27 and adherence to PFMT27 30 for women with UI. On the contrary, some studies29 31 found no significant differences in self-reported symptom severity, condition-specific QoL or adherence to PFMT between the WBIs and control groups.
Currently, there are five systematic reviews of WBIs for women with UI. Two reviews only focus on the effects of mobile applications on the UI. Among them, Widdison et al24 included four trials, and Leme Nagib et al21 included three RCTs, both of which indicated that WBIs had significant improvements in self-reported symptom severity, condition-specific QoL and adherence to PFMT. Yet, both reviews included male and female UI patients but did not report outcomes separately for women. Similarly, Hou et al32 included six RCTs and only assessed the effectiveness of mobile application-delivered PFMT for stress UI in women and reported significant improvement in self-reported symptom severity, condition-specific QoL, adherence to PFMT and the global impression of improvement. Nevertheless, the certainty of the findings from the above three reviews is limited, as the authors only provided a narrative description of the results but did not conduct any quantitative syntheses, mainly due to the limited number of studies for each outcome. In another systematic review and meta-analysis conducted by Huang et al22 (n=7 RCTs), the effects of telemedicine for UI in women were investigated, with the results indicating reductions in self-reported symptom severity, anxiety and depression, as well as improvements in QoL, self-efficacy of PFMT and the global impression of improvement for the targeted population. However, caution should be exercised when interpreting the results of this review, as it conflated early mobile technologies (such as telephone calls) with web-based technologies. Interestingly, the results of Papanikolaou et al’s systematic review and meta-analysis23 (n=10 RCTs) contradicted the above four reviews by showing no significant difference in self-reported symptom severity, condition-specific QoL or adherence to PFMT between WBIs and the control group. On the whole, the findings across existing systematic reviews pertaining to this topic are lacking in congruity, with certain outcomes such as pelvic floor muscle contractility, incontinence episode frequency, usage rate of incontinence aids and satisfaction with the intervention being rarely evaluated. Moreover, all of these reviews incorporated a restricted quantity of primary studies (n≤10), while an increasing number of RCTs27–31 concerning this topic were being published after them, which could offer novel evidence. Accordingly, the effects of WBIs on women with UI need further exploration.
Objectives
The purpose of this systematic review and meta-analysis is to investigate the effectiveness of WBIs for women with UI based on all available evidence from RCTs. Specifically, our proposed review seeks to answer the following questions: (a) Whether WBIs can effectively mitigate self-reported symptom severity, improve condition-specific QoL and increase adherence to PFMT (primary question); (b) Whether specific types of intervention format, intervention interactivity and main technology have beneficial effects on these outcomes (secondary question 1) and (c) Are WBIs effective on other extensive secondary outcomes among women with UI (secondary question 2).
Methods and analysis
Registration
This systematic review and meta-analysis has been prospectively registered on the platform of the International Prospective Register of Systematic Reviews (PROSPERO). The registration number is CRD42023435047. Any future changes to the study protocol will be registered as amendments.
Eligibility criteria
The PICOS approach will be used for eligibility criteria. The details are described as follows:
P (population)
Female adults diagnosed with any type of UI will be considered eligible. However, women affected by UI arising from non-urinary tract factors will be excluded, such as cancer or radiotherapy-induced symptoms, neurological, cognitive or psychological disorders, and diseases hindering independent mobility. Also, studies that involved women with various types of lower urinary tract symptoms but did not report the data specific to women with UI separately will be excluded.
I (intervention)
The intervention should be in a digital format using any web-based technologies, including but not limited to websites and mobile applications, with or without an intravaginal digital device connection. Nevertheless, studies will be excluded if they solely use WBIs to observe the maintenance effects of previously administered health interventions, compare different types of programme-specific or module-specific WBIs, involve face-to-face components in addition to the same routine care received by the control group, or do not conduct real WBIs (such as by adopting telephone call, short message or digital video disk for intervention).
C (control)
The control group can be usual care, a waitlist, no treatment or minimal WBIs (such as hyperlinks to brief informational websites or an application that delivers concise information).
O (outcome)
The primary outcomes are self-reported symptom severity, condition-specific QoL and adherence to PFMT. Secondary outcomes will include pelvic floor muscle contractility evaluated by digital palpation (focusing on four out of the six domains of the PERFECT assessment scheme: power, endurance, repetition and fast), incontinence episode frequency, urine leakage volume (measured via the pad-weighing test), usage rate of incontinence aids, the global impression of improvement, disease-related knowledge, self-efficacy of PFMT, mental health (anxiety and depression) and satisfaction with intervention. Studies that assess at least one of the aforementioned outcomes will be recognised as qualified.
S (study design)
This systematic review will only include RCTs with full-text research papers available. Studies published in English and Chinese will be included.
Information sources and search strategy
The three-step approach to literature search recommended by the Joanna Briggs Institute will be used to identify studies that are relevant to the review questions. Step 1: An initial search has been conducted only on PubMed and CNKI, followed by an analysis of the text words in the titles and abstracts of the retrieved publications, as well as the corresponding Medical Subject Heading (MeSH) terms to describe them. Step 2: Based on the results of the initial search modification and additional keywords, we will collaborate with an academic librarian to develop customised search strategies for each electronic database. Moreover, the search strategies of two systematic reviews33 34 published by our research team were also referred to when developing the current search strategy. A combination of MeSH terms and free-text keywords will be applied where appropriate to represent the definitions of WBIs, UI and RCT if possible. Table 1 shows the detailed search strategies of PubMed and CNKI. 10 electronic databases are anticipated to be comprehensively searched from their inception to 1 May 2024, by 2 authors independently, including 6 English databases (PubMed, Embase, the Cochrane Library, Web of Science, PsycINFO and CINAHL) and 4 Chinese databases (CNKI, Wanfang Data, VIP and SinoMed). Step 3: Google Scholar and Baidu Library sources will be searched for grey literature. The reference lists of all eligible articles and relevant reviews will be manually examined to expand the scope of our search and retrieve additional eligible studies.
Literature search strategy
Screening and selection procedures for eligible studies
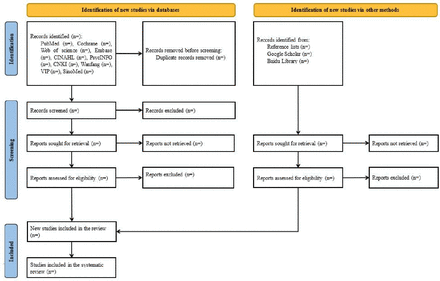
EndNote V.X9 will be used to manage the retrieved studies, where duplicate references will be identified and removed by using the automated ‘Find Duplicates’ function. A two-stage process will be used to determine the eligibility of each publication. In the first stage, two independent reviewers will screen the titles and abstracts of the papers retrieved and an article will be temporarily retained if either of the two reviewers considers it to be potentially eligible for inclusion. The authors of potentially eligible studies for which the full text is not available will be contacted via email to seek either the full text or their research data. In the second stage, the full texts of the remaining studies will be read. Discussions will be used to reach a consensus if there are any discrepancies at the full-text level. Consultation by a third reviewer will be performed if necessary when an agreement cannot be reached through discussion alone. The article selection process and reasons for excluding studies will be shown in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (figure 1).
Flow diagram of the article selection process.
Data abstraction
Two different reviewers will extract the data into a purpose-built, structured sheet, and any discrepancies will be resolved through arbitration and consensus among the members of our research team. Beforehand, the data extraction sheet will be first piloted with a subsample of included studies and then revised and refined as necessary. If studies report outcomes at different time points, the outcomes evaluated at immediate postintervention termination will be extracted, for the effect sizes observed at the end of the intervention are considered the most pertinent measures of potential benefits. The following information will be collected from eligible studies:
The general information of the study: first author, study country and publication year.
The baseline characteristics of participants: type of UI, diagnostic criteria, mean age and sample size.
The details of the WBIs: the intervention’s name, detailed regimen, duration, format (personalised or non-personalised), interactivity (interactive or non-interactive) and main technology.
The intervention regimen of the control group.
The details of the outcome: data on outcomes, measurement tools, between-group difference (±), evaluation time points.
Adverse events.
The attrition rate.
Quality appraisal
The quality of the included studies will be assessed independently by two investigators through the Cochrane Collaboration tool.35 This tool consists of seven items: random sequence generation (selection bias), concealment of allocation (selection bias), blinding of the subjects and personnel (performance bias), outcome assessor blinding (detection bias), completeness of follow-up (attrition bias), selective reports (reporting bias) and other biases. Each item will be rated as ‘low’, ‘unclear’ or ‘high’ risk of bias. Any disagreements will be settled by discussion with a third reviewer.
Statistics analysis
Data synthesis
Where possible, meta-analyses will be conducted to combine the data. We will use mean differences with 95% CIs for continuous variables that were assessed with the same instrument and standardised mean differences with 95% CIs when a similar outcome was measured with different instruments. The mean differences/standardised mean differences between the intervention and control groups will be calculated based on the mean change (mean change=mean after−mean baseline) and the corresponding SD change (SD change=√ [SD2 baseline + SD2 after−(2×r×SD baseline×SD after)]).36 r is the correlation between the matched pairs of preintervention and postintervention assessments. In fact, it has been demonstrated that there are no significant differences in the calculated effect size between correlations=0, 0.3 and 0.7.36 In this study, a r of 0.5 was assumed for all analyses as it is the most commonly used for calculation.37 38 The effect size of standardised mean differences is reported as small (<0.2), moderate (0.2–0.8) or large (>0.8) based on Cohen’s definition.39 Regarding binary variables, we will choose relative risks with 95% CIs as the point estimate, with the cut-off values of 1.22, 1.86 and 3.00 denoting small, medium and large effects, respectively.40 The Mantel-Haenszel method will be employed to combine dichotomous outcome data, and the inverse variance method will be employed for pooling continuous outcome data. All statistical analyses will be performed using Stata, V.12.0 (StataCorp). Statistical significance will be defined as a p<0.05. For outcomes that cannot be quantitatively synthesised in a meta-analysis because of insufficient data (less than three studies report the data), high heterogeneity of effect measurement tools, or other reasons, a narrative approach will be used for describing and summarising.
Assessment of heterogeneity
The degree of heterogeneity across studies will be assessed using both the χ2 test and the I2 test. According to the Cochrane Handbook, Higgins I2 from 0% to 40% means that there is insignificant heterogeneity, from 30% to 60% indicates that there is moderate heterogeneity, from 50% to 90% represents that there is substantial heterogeneity and >75% manifests that there is high heterogeneity.39 A fixed-effects model will be chosen for analysis only when no substantial heterogeneity exists (p≥0.1 and I2≤50%), whereas a random-effects model will be used if there is significant heterogeneity (p<0.1 and I2>50%) since it can provide more cautious summary effect estimates and is preferred when there is unexplained heterogeneity across studies.39
Sensitivity analysis
Sensitivity analyses will be performed by excluding one study at a time to determine if any individual study has a significant impact on the merged results. A comparison will be made between the merged results prior to the modifications and the adjusted results in order to identify the potential sources of heterogeneity.
Publication bias
Publication bias assessment of an outcome will be detected by using the funnel plot and Egger’s test when the number of included studies reaches or exceeds 10.
Subgroup analyses
Three subgroup analyses will be carried out to investigate the influence of the type of format (personalised and non-personalised), type of interactivity (interactive and non-interactive), and main technology (such as mobile applications and websites) on the effects of WBIs on primary outcomes, thereby attempting to explore an optimal WBIs regimen for women with UI.
Patient and public involvement
This review will be based solely on publicly accessible studies. Thus, no patient or member of the public will be directly involved in the design, implementation, reporting or dissemination of the study.
Validity, reliability and rigour
This study protocol was reported according to the PRISMA Protocol statement guidelines (PRISMA-P) (see online supplemental material). We will conduct and report the systematic review strictly in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and the guidelines of the PRISMA statement to ensure its validity, reliability and rigour.
Supplemental material
Discussion
As one of the most common chronic diseases among women, UI can cause many physical, mental and social discomforts for individuals and result in huge financial burdens on families and society as a whole.6–10 Although the traditional face-to-face mode of UI management is effective,12 it demands substantial human and financial inputs.19 20 WBIs have drawn great attention from the medical and hygiene fields due to their advantages of high accessibility and efficiency.17 25 26 Recently, some researchers have attempted to use WBIs to manage UI for women, but the effectiveness of WBIs among this crowd has remained inconclusive,27–31 and even the existing relevant systematic reviews failed to arrive at a consensus on this matter,21–24 32 which impedes clinical decision-making and limits the widespread application of WBIs. Accordingly, this paper presents a protocol for a systematic review and meta-analyses that will summarise the related evidence by systematically reviewing previous RCTs regarding the effectiveness of WBIs in women with UI. It is anticipated that the findings of the future systematic review will allow for more considerate and insightful recommendations when advising UI women with effective management strategies.
With regard to selecting outcomes, since self-reported symptom severity and condition-specific QoL can appropriately reflect the impacts of UI on women’s physical health, mental health, and social engagement and play crucial roles in determining whether additional treatments are warranted,41 and adherence to PFMT represents a fundamental element of the effectiveness of a PFMT programme,14 the future systematic review will adopt self-reported symptom severity, condition-specific QoL and adherence to PFMT as the primary outcomes. In the meantime, a wide range of secondary outcomes will also be evaluated to enhance the overall comprehension of the WBIs’ effectiveness in managing UI for women. Consequently, the findings of this review will provide more solid evidence and comprehensive references on whether WBIs should be extensively suggested in the future for women’s UI management in clinical settings. Furthermore, we will also collect information about the attrition rate of participants and adverse events that happened during WBIs in the data abstraction process to find out the possible disadvantages of WBIs, although they were not set as the secondary outcomes for this review, which may provide useful information for improving the WBIs regimen.
Additionally, subgroup analyses based on the interactivity, format and main technology of WBIs will be conducted. It is expected that the corresponding results can aid healthcare providers in developing and implementing an optimal WBI programme for UI women, thereby generating maximum benefits for the intended audiences, medical staff and other related stakeholders.
Nonetheless, we have recognised some potential limitations of this review. First, the use of web-based technologies in healthcare is a developing field, so there might be limited studies available on this topic. Second, some studies in other languages can be overlooked because this review will only include RCTs published in English and Chinese. Third, heterogeneity will exist inevitably in meta-analysis in terms of the variation of clinical and methodological characteristics. For instance, the diversity of UI types and the duration of WBI characteristics will lead to heterogeneity. Thus, we intend to carry out leave-one-out sensitivity analyses to evaluate the stability of the pooled results and identify potential sources of heterogeneity.
Ethics and dissemination
Ethical approval and participant consent will not be required since this study will consist of a secondary analysis of published evidence and not contain any private information about participants. The findings will be disseminated via a peer-reviewed journal or an international conference.
Ethics statements
Patient consent for publication
Acknowledgments
The authors thank the students who took the time to become involved in this study.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
XX and PG are joint first authors.
XX and PG contributed equally.
Contributors XX: conceptualisation, visualisation, investigation, writing–original draft. PG: conceptualisation, visualisation, writing–review and editing. PX: investigation, writing–review and editing. DDC: investigation, writing–review and editing. WC: formal analysis, writing–review and editing. HW: formal analysis, writing–review and editing. YJ: formal analysis, writing–review and editing. XW: funding acquisition, writing–review and editing. WZ: funding acquisition, writing–review and editing. FX: funding acquisition, writing–review and editing. RZ: funding acquisition, writing–review and editing. MM: funding acquisition, writing–review and editing. SF: conceptualisation, visualisation, writing–review and editing.
Funding This study is an externally funded research project supported by the Science Research Foundation of the National Health Commission in China-Zhejiang Provincial Health Major Science and Technology Project (WKJ-ZJ-1925), the China Scholarship Council (No.202306320022), the Zhejiang University Academic Award for Outstanding Doctoral Candidates (grant number: 2023095) and the Zhejiang Province Medical and Health Technology Plan Project (2023KY818).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.