Article Text
Abstract
Introduction The classic way of diagnosing prostate cancer (PCa) is by conducting the 12-core systematic biopsy (SB). However, it has a low detection rate for clinically significant PCa (csPCa) and can lead to the detection of clinically insignificant PCa (cisPCa). Although MRI-transrectal ultrasound (MRI-TRUS) fusion targeted biopsy (TB) can effectively improve the detection rate of csPCa, it may still miss some cases. Therefore, we propose using a combination of TB and SB methods to enhance the detection rate of csPCa while minimising the detection rate of cisPCa.
Methods and analysis This study is a prospective, single-centre investigation that aims to assess and compare the detection rate of csPCa using MRI-TRUS fusion TB combined with SB versus TRUS 12-core SB alone. Biopsy-naïve men with suspected PCa will be subjected to multiparametric MRI. Patients with Prostate Imaging Reporting and Data System (V.2.1) score ≥3 will be enrolled in the TB-SB combination group. The sample size is established as 660 participants, considering a 10% drop-out rate. The primary outcome is the detection rate of csPCa in men without prior biopsy using MRI-TRUS fusion TB combined with the standard TRUS-guided 12-core SB method. CsPCa will be defined as International Society of Urological Pathology Grade ≥2.
Ethics and dissemination This study has been approved by the Ethics Committee at the Shanghai Tenth People’s Hospital, an affiliated hospital of Tongji University School of Medicine. The research results will be published in a peer-reviewed international journal.
Trial registration number ChiCTR2000036089.
- Prostatic Neoplasms
- Magnetic Resonance Imaging
- Biopsy
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study uses the patients’ histopathological examination results, thus restricting confounding factors and enhancing comparability.
The systematic biopsy (SB) operator is unaware of the location of MRI targets, ensuring that the accuracy of SB is not influenced by the multiparametric MRI results.
Patients with scores 1–2 will not be considered prostate cancer-free, and the findings of this study will not apply to them.
Since this study will be conducted in a single centre, the results may lack generalisability.
Introduction
Recently, the incidence of prostate cancer (PCa) has been progressively increasing, making it the most frequently occurring malignant tumour of the urogenital system.1 Therefore, timely detection of PCa has become increasingly imperative.2 3 Currently, prostate biopsy is the standard method for diagnosing PCa.4 5 Studies show that the risk of progression and prognosis varies among the different stages of PCa. Notably, clinically insignificant PCa (cisPCa), which is a low-grade cancer, presents a negligible risk of progression and metastasis and has a minimal impact on patient survival.6–8 On the other hand, clinically significant PCa (csPCa) presents a high risk of progression and metastasis, often leading to treatment resistance and posing a severe threat to patient survival.7 Thus, the key to the early diagnosis of PCa is the detection of csPCa.
Currently, systematic biopsy (SB) guided by transrectal ultrasound (TRUS) is considered the classic method for the early diagnosis of PCa. Previous studies have shown that SB has a low csPCa detection rate, while also detecting cisPCa, thus leading to overdiagnosis and overtreatment.9 Recently, the advancement of multiparametric MRI (mpMRI) technology has greatly improved the sensitivity and specificity in detecting csPCa.10 11 Therefore, fusing images of suspicious lesions found on mpMRI with TRUS enables the implementation of targeted biopsy (TB) for the suspicious lesions. Studies show that MRI-TRUS fusion-guided TB can effectively improve the detection rate of csPCa.10 However, using TB alone could result in a missed diagnosis of csPCa.12 13 Thus, the combined biopsy approach could potentially serve as a viable solution to address the aforementioned issues. Software fusion is one of the most commonly used methods for MRI-TRUS fusion in PCa.14 Therefore, we believe that combining SB and TB under software fusion will not only improve the detection rate of csPCa but also reduce the detection rate of cisPCa.
Currently, there are two main types of biopsy approaches for prostrate biopsy, namely the transrectal and transperineal routes.15 Transperineal prostate biopsy is considered superior to transrectal biopsy by our hospital’s cancer team. Our hospital was the first in China to conduct a randomised controlled trial comparing the two routes, and the results showed that though the overall detection rate of PCa was similar between the transperineal and transrectal routes, the safety of the former was significantly superior to that of the latter.16 Moreover, the transrectal approach is more likely to miss detecting certain tumours in the apex and transitional zone compared with the transperineal approach.15 17
In this study, we will use the transperineal route with the mpMRI-TRUS software fusion mode to compare the MRI-TRUS fusion TB and SB method with SB alone for the detection of csPCa. The implementation of this study is expected to clarify the contributions of TB and SB while providing high-level evidence to establish a new effective and precise model for prostate biopsy.
Methods and analysis
Trial design
This single-centre prospective study will be conducted at the Shanghai Tenth People’s Hospital affiliated with Tongji University School of Medicine, Shanghai, China. This study aimed to evaluate and compare the detection rate of csPCa between MRI-TRUS fusion TB and SB and the standard TRUS 12-core SB method in patients with elevated prostate-specific antigen (PSA) levels. The research process is illustrated in figure 1 and summarised in table 1. Patient recruitment for the study commenced on 1 October 2020, and the research activities, including biopsies and follow-up examinations, are scheduled to conclude on 1 December 2024. We will conduct mpMRI examinations for patients with suspected PCa, who seek medical attention at our institution. Suspicious lesions identified on mpMRI will be assigned a Prostate Imaging Reporting and Data System (PI-RADS, V.2.1) score.11 Patients with PI-RADS score ≥3 will undergo transrectal TB combined with SB after enrolment and providing written informed consent. Biopsy specimens will be sent to the pathology department for analysis within 24 hours. Any adverse events (AEs) experienced by patients will be recorded 24 hours and 4 weeks after the biopsy.
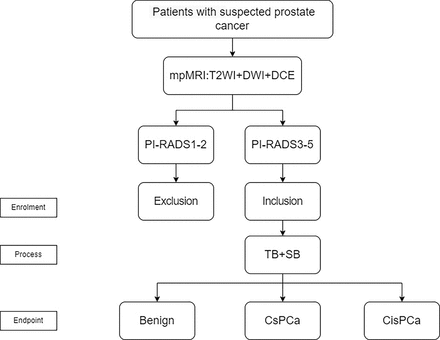
Trial flow chart. Biopsy-naïve men with suspected prostate cancer will be subjected to multiparametric MRI. The mpMRI should consist of at least three sequences: T2WI, DWI and DCE. For each suspicious lesion identified in the mpMRI, we assign a PI-RADS score (ranging from 1 to 5, with higher scores indicating an increased likelihood of csPCa). Patients with PI-RADS scores of 3–5 will be included in the study. Enrolled patients will undergo both targeted biopsies and systematic biopsies, followed by the collection of corresponding pathology reports for further analysis. The csPCa will be defined as Gleason score ≥7 (3+4), corresponding to Gleason Grade ≥2. cisPCa, clinically insignificant prostate cancer; csPCa, clinically significant prostate cancer; DCE, dynamic contrast-enhanced; DWI, diffusion-weighted imaging; mpMRI, multiparametric MRI; PI-RADS, Prostate Imaging Reporting and Data System; SB, systematic biopsy; T2WI, T2-weighted imaging; TB, targeted biopsy.
Participant timeline in the study
Outcomes
The primary outcome is to evaluate and compare the detection rates of csPCa in patients with elevated PSA levels who are undergoing their first biopsy. This will be accomplished by comparing MRI-TRUS fusion TB combined with SB and standard TRUS 12-core SB, thus providing an effective detection method for csPCa.
The secondary outcomes are as follows:
Detection rate of cisPCa in patients.
Overall detection rate of PCa in participants who underwent MRI-TRUS fusion TB combined with SB versus those who underwent only SB.
The consistency level between the biopsy pathological findings and postoperative pathological findings will be evaluated in patients confirmed with PCa through biopsy and subsequently undergoing radical prostatectomy.
Patient and public involvement
There was no involvement of patients and the public in this study’s design or implementation.
Patient population
Patients with elevated PSA levels and no prior biopsy will be recruited if they fulfil the eligibility criteria outlined in box 1. Patients who agree to participate in this study will be fully informed of the risks and benefits involved and will be required to sign an informed consent form. Patients may withdraw from the study at any time without reason, but they will be informed that regular follow-up and treatment will continue as usual following withdrawal.
Inclusion and exclusion criteria for the study
Inclusion criteria
Aged between 35 and 85 years old.
The serum total PSA>=10 ng/mL, the PSA is between 4 and 10 ng/mL while the ratio of free to total PSA was <0.16.
DRE normal or abnormal DRE with lesion confined to the prostate.
The PI-RADS of mpMRI scan is 3–5.
The participant is able to finish the examination in this clinical trial.
Signed the informed consent.
Exclusion criteria
The PI-RADS of mpMRI scan is 1 or 2.
The patients had received prostate biopsy in a history.
The patients had received treatment for prostate cancer.
The patients have contraindications for mpMRI scan, such as claustrophobia, implantation of heart pacemaker, metallic medical instrument and GFR≤50 mL/min.
The patients have contraindications for prostate biopsy, such as recto fistula and active urinary tract infection.
The participant is unable to finish the examination in this clinical trial.
The patients refused to finish the trail according the protocol after enrolled.
mpMRI, multiparametric MRI; PI-RADS, Prostate Imaging Reporting and Data System; PSA, prostate-specific antigen; DRE, digital rectal examination; GFR, glomerular filtration rate.
Multiparametric MRI
All enrolled patients should have undergone mpMRI examination according to the following specifications. The mpMRI field strength should be set at 3.0 Tesla with transabdominal phased-array coils. The image acquisition will strictly adhere to the technical standards outlined in PI-RADS V.2.1. The mpMRI sequences will include T2-weighted imaging (T2WI), dynamic contrast-enhanced (DCE) imaging and diffusion-weighted imaging (acquired b-values of 0, 100, 800 and 1500 s/mm) with apparent diffusion coefficient. We will perform T2WI in the axial, coronal and sagittal planes with a slice thickness of 3 mm and using three-dimensional axial acquisitions. A gadolinium-based contrast agent will be administered intravenously at a rate of 2 mL/s for DCE, with the dosage determined based on the patient’s body weight. Suspicious pathological changes identified on mpMRI will be evaluated and scored by two proficient uroradiologists using the PI-RADS (V.2.1). In the event of discordance in the ratings provided by the two physicians, a medical practitioner with a higher professional designation shall serve as an arbitrator. The PI-RADS score is in the range of 1–5, with higher scores indicating an increased likelihood of csPCa.
Biopsy
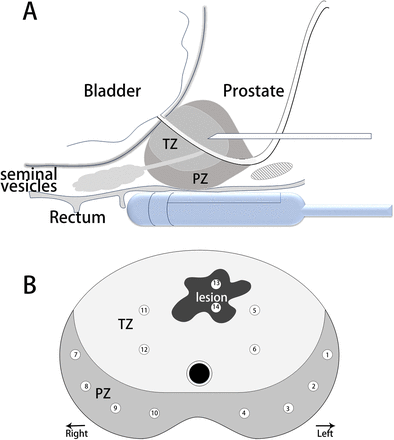
To minimise the bleeding and swelling in the prostate resulting from SB, we will initially perform the fusion TB followed by the standard TRUS 12-core SB. Two urologists with over 10 years of relevant experience will conduct all biopsies. The MRI-TRUS fusion imaging and biopsies will be conducted using the MyLab Twice US scanner (Esaote, Genoa, Italy), which is equipped with fusion software, a biplanar transrectal transducer operating at a frequency range of 6–9 MHz (TRT33; Esaote), and a real-time transducer navigation system. With the assistance of the operator, the system in navigation mode can identify the target on the MRI and register the acquired MRI-TRUS fusion image in the MRI-TRUS fusion workstation to determine the biopsy target on the MRI. Subsequently, the first urologist will manually perform 2–4 needle biopsies for each biopsy target (figure 2).The second urologist will perform TRUS 12-core SB without the MRI results (figure 2). This approach will prevent the urologist from intentionally or unintentionally approaching the location of the lesion with PI-RADS scores 3–5 during SB, thus ensuring the procedural standardisation of the biopsy.
(A) Illustration of combined biopsy. (B) Twelve-region template-guided prostate biopsy and biopsy biopsy with 2–4 needles for the location of the lesions with PI-RADS scores 3–5. PI-RADS, Prostate Imaging Reporting and Data System; PZ, peripheral zone; TZ, transition zone.
Histology
All biopsy specimens will be properly labelled and promptly sent to the pathology department within 24 hours. The labelling information will include the biopsy site and whether TB or SB was performed to obtain the sample. The analysis of the biopsy samples will include the determination of the Gleason scores based on the International Society of Urological Pathology convention.18 An independent evaluation by a second pathologist will be conducted to ensure the accuracy and consistency of the pathological reports, and any disagreements will be resolved through further review. If the two pathologists disagree, the pathological diagnosis by the pathologist with a higher professional title will prevail. The pathological report will include information on the tissue type, Gleason score and proportion of cancerous tissue for each biopsy sample. For patients who have undergone radical prostatectomy for PCa at our institution, the postoperative pathology report of the PCa will be documented. The csPCa will be defined as Gleason score ≥7 (3+4), corresponding to Gleason grade ≥2.
Follow-up
Patients will resume their normal clinical follow-up or treatment protocols. We will collect information regarding any AEs or complications that occur in patients during the procedure, within 24 hours postoperatively and after 4 weeks. For patients who undergo radical prostatectomy for PCa, further follow-up will be conducted for the postoperative pathological reports.
Statistical analysis
Descriptive statistics will be used to report continuous variables, including means and SDs or medians and IQRs. Categorical variables will be presented as frequencies and proportions. The primary hypothesis is that TB combined with SB improves the detection rate of csPCa compared with SB alone. Adjusted Wald intervals will be used to calculate the CI of the cancer detection rate and the difference in the cancer detection rate between the biopsy methods. The threshold for the difference in the detection rate of csPCa between the two diagnostic methods is set at 5%, with the equivalence test threshold set at Δ+5%. If the difference in the detection rate of csPCa between the two methods is >5%, we can conclude that TB combined with SB is more effective than SB alone. All reported p values in this trial are two tailed.
Sample size
Multiple research studies have demonstrated that the detection rate for csPCa with mpMRI-TRUS fusion TB is 31%–52%, whereas that with standard TRUS 12-core SB is 26%–39%.19 20 Furthermore, previous research conducted by our PCa team indicated a detection rate of 32% for csPCa with SB at our medical centre, which represents the general population since our centre is a tertiary hospital in northern Shanghai. Therefore, it can be assumed that the detection rate of csPCa with MRI-TRUS fusion-TB combined with SB will be 40%, whereas that with SB alone will be 30% in this study. Considering a bilateral test level α-value of 0.05 and a detection power level (1−β value) of 0.80, the threshold for the difference in the detection rate of csPCa between the two diagnostic approaches was set at 5%, that is, the equivalence test threshold was set at Δ+5%. As this study involves a single sample, 594 participants will be required for statistical analysis, accounting for an anticipated drop-out rate of 10% (λ). Therefore, the study aims to recruit 660 participants in total.
Complications and AEs
All participants will be monitored for AEs during the biopsy procedure, 24 hours postoperatively and after 4 weeks. All complications or AEs will be recorded in the medical records and submitted to the ethics committee. We will use the Common Terminology Criteria for Adverse Events (V.5.0) to evaluate the AEs. The common AEs expected include pain, haematuria, haematospermia, erectile dysfunction, urinary incontinence and febrile urinary tract infections. Serious AEs include death, life-threatening situations, hospitalisation and permanent or severe disability and functional impairment.
Data collection
Information such as demographic data (age, height, weight and body mass index), PSA levels, mpMRI categorical variables (PI-RADS score, prostate volume and suspicious lesion volume), prostate biopsy pathology and postradical prostatectomy pathology will be collected from the patient’s registration information, hospital discharge records, 4-week follow-up records after discharge and pathological status. The medical reports of each participant will be completely recorded in the database established in EXCEL software so that the data can be easily accessed and managed. The data collected in the database will only be used for this study and not for other purposes.
Monitoring
This study has been registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2000036089). An independent data monitoring committee will be established, which will be responsible for supervising the objectivity and accuracy of the research data. The committee will not participate in patient recruitment, examinations, operations or result analysis processes. Additionally, if potential risks that affect patient safety are identified, the committee will contact the researchers promptly and consider modifying the research protocol or suspending the study. Furthermore, this study will be supervised by the higher authority, and relevant departments of the hospital, and data management and security reports will be submitted to the ethical committee every 6 months. The data can be shared within 6 months after completion and will be available on reasonable request via email.
Study period
The study received approval from the ethic committee of Shanghai Tenth People’s Hospital on 3 February 2020. Participant recruitment began on 1 October 2020. The study is scheduled to conclude all patient follow-ups and data analysis by 1 December 2024.
Discussion
Since the advent of TB techniques, there has been ongoing debate regarding the choice of the biopsy method. SB is a classic approach and has been consistently used as a benchmark for comparison with various other approaches. Several studies have demonstrated that TB and SB exhibit similar detection rates for PCa; however, TB has a higher sensitivity for csPCa than SB.12 21–23 A large retrospective study conducted by Ahdoot et al revealed an overall PCa detection rate of 51.5% with TB compared with 52.5% with SB, while the csPCa detection rate was 37.8% with the former and 30.9% with the latter.12 Thus, TB manifests a clear advantage in diagnosing csPCa, and the need for SB remains controversial. Eklund et al support performing SB in men with positive MRI results.24 The study by Brisbane et al indicates that all cancer lesions are not located within the areas detected on MRI, and only performing TB is insufficient.25 Deniffel D et al suggest using the PI-RADS score to determine the need for SB, and patients with a score of 5 should not be subjected to the SB approach.26 27 The GÖTEBORG-2 study demonstrates a 54% reduction in the detection of Gleason 3+3 cancer with TB alone; therefore, considering all the factors, SB should be avoided.28
This prospective study aims to assess the efficacy of the combined TB and SB approach to establish a rational biopsy method. By separating TB and SB during the process, we ensure that the accuracy of SB is not influenced by the mpMRI results. Therefore, our study can demonstrate the effectiveness of performing SB in patients with visible lesions on mpMRI. It provides the corresponding evidence-based medicine facts on whether these patients should undergo SB.
This study undeniably has some limitations. First, it will be conducted at a single centre, potentially limiting the generalisability of the findings. Second, the utilisation of software fusion for TB introduces the possibility of computational inaccuracies. Furthermore, this study solely focuses on patients with PI-RADS scores 3–5 on mpMRI scans, rendering it insufficient to provide a reference value for patients with suspected PCa having PI-RADS scores 1–2.
Thus, our prospective study will elucidate the contributions of the combined biopsies technique, providing an effective detection method for csPCa.
Ethics and Dissemination
This clinical trial shall abide by the principles stipulated in the Declaration of Helsinki. Approval for this study has been granted by the Ethics Committee of Shanghai Tenth People’s Hospital. All patients who voluntarily agree to participate in this study will be required to sign a written informed consent form that explains in detail the nature of their participation. The form will be given to the patients after a comprehensive explanation, and they will be allowed ample time to consider and seek counsel on the matter (at least 24 hours). The researchers will provide contact information if the patients are interested in understanding more about this study. The findings of this research will be published in a peer-reviewed journal.
Ethics statements
Patient consent for publication
References
Footnotes
WL and AK are joint first authors.
X @BinYang9
Correction notice This article has been corrected since it was first published. PSA values in Box 1 has been updated to 'PSA>=10ng/mL'.
Contributors WL: writing original draft, data collection and data analysis. AK: writing review and editing, data collection and patient care. DS: patient care and supervision. YH: patient care and data collection. SD: patient care and data collection. KZ: patient care and supervision. GX: patient care and supervision. BZ: patient care and data collection. SM: patient care and supervision. CG: patient care and data collection. XY: supervision and patient care. QW: patient care. DH: supervision and patient care. BY: protocol design, funding aquisition, writing review and editing, methodology, supervision, ethical submission and patient care.
Funding This study was supported by the grants from Shanghai Tenth People's Hospital(2023YJXYSB004, SHYCS12), Shanghai Hospital Development Center (SHDC2020CR6007), the National Natural Science Foundation of China (31570993) and the Wu Jieping Medical Foundation (320.6750.2020-14-7).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.