Article Text
Abstract
Introduction With the growing emphasis on swift recovery, minimally invasive thoracic surgery has advanced significantly. Video-assisted thoracoscopic surgery (VATS) has seen rapid development, and the double-lumen tube (DLT) remains the most dependable method for tracheal intubation in VATS. However, hypoxaemia during DLT intubation poses a threat to the perioperative safety of thoracic surgery patients. Recently, transnasal high-flow nasal oxygen (HFNO) has shown promise in anaesthesia, particularly in handling short-duration hypoxic airway emergencies. Yet, its application in the perioperative period for patients undergoing pulmonary surgery with compromised cardiopulmonary function lacks evidence, and there are limited reliable clinical data.
Methods and analysis A prospective, randomised, controlled, single-blind design will be employed in this study. 112 patients aged 18–60 years undergoing elective VATS-assisted pulmonary surgery will be enrolled and randomly divided into two groups: the nasal high-flow oxygen group (H group) and the traditional mask transnasal oxygen group (M group) in a 1:1 ratio. HFNO will be used during DLT intubation for the prevention of asphyxia in group H, while conventional intubation procedures will be followed by group M. Comparison will be made between the two groups in terms of minimum oxygen saturation during intubation, hypoxaemia incidence during intubation, perioperative complications and postoperative hospital days.
Ethics and dissemination Approval for this study has been granted by the local ethics committee at Shenzhen Second People’s Hospital. The trial results will be disseminated through peer-reviewed journals and scientific conferences.
Trial registration number NCT05666908.
- high-flow nasal oxygenation
- double-lumen tube
- thoracoscopy pulmonary surgery
- hypoxemia
- thoracoscopic surgery
- transnasal humidified rapid insufflation ventilatory exchange (THRIVE)
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- high-flow nasal oxygenation
- double-lumen tube
- thoracoscopy pulmonary surgery
- hypoxemia
- thoracoscopic surgery
- transnasal humidified rapid insufflation ventilatory exchange (THRIVE)
STRENGTHS AND LIMITATIONS OF THIS STUDY
Numerous thoracoscopic surgeries are conducted annually at our hospital, allowing for a readily available sample size.
The utilisation of video cameras to document our operating room procedures guarantees the precision of our data recording.
All procedures in the operating room will be executed by the same anaesthesiologist.
This protocol does not include some populations that could potentially benefit from high-flow nasal oxygen.
This study is limited to a single centre.
Introduction
Hypoxaemia significantly jeopardises patient safety during surgery, as per the American Society of Anesthesiologists (ASA) Closed Claims Analysis Report. The report indicates that respiratory issues account for a notable portion of death and brain injury claims within the ASA Closed Claims Database.1 In a retrospective study with 95 407 electronic anaesthesia records, 6.8% of patients experienced hypoxaemic events (SpO2<90%), and 3.5% faced severe hypoxaemic events (SpO2<85%) persisting for 2 consecutive minutes or more.2 Crucially, over 70% of hypoxaemic incidents occurred during anaesthesia induction.2 3
Double-lumen tubes (DLTs) are commonly employed in thoracic surgery for procedures requiring one-lung ventilation (OLV).4–6 Throughout this process, airway intubation advances through specific phases, including preoxygenation, induction, hypoventilation, apnoea and tracheal intubation. In contrast to traditional single-lumen tubes, DLTs exhibit increased thickness and rigidity, introducing intricacies to the intubation procedure. This intricacy requires a more detailed positioning with fibreoptic bronchoscopy (FOB), leading to extended apnoea duration during intubation. In previous studies, the intubation time for a DLT could be as long as 4.6–9.5 min.7 The intricate process of DLT intubation, notably with its substantial extension of apnoea duration, unquestionably elevates the risk of hypoxaemia and associated respiratory complications for patients.
As a burgeoning form of respiratory support, high-flow nasal oxygen therapy (HFNO) is increasingly prevalent in anaesthesia.8 9 HFNO sustains apnoeic oxygenation through mechanisms such as continuous positive airway pressure and gas exchange via flow-dependent deadspace flushing.10 11 In comparison to mask oxygenation, HFNO holds the advantage of not requiring interruption during laryngoscopy. A growing body of evidence indicates that HFNO usage can extend the safe apnoea time during intubation.10 12 13 Consequently, it is recommended for apnoeic oxygenation during intubation in patients at a high risk of difficult airway management.14 15
To our knowledge, there are few studies investigating the application of HFNO in DLT intubation procedures in thoracic surgery. A case report from (CRF) 2021 described the effective insertion of a DLT in a patient with a bronchopleural fistula, facing challenges in tolerating the supine position. The DLT was successfully placed following rapid sequential induction while the patient was in a frontal sitting position, with the assistance of HFNO.16 This indicates the potential usefulness of HFNO in the context of more intricate DLT intubations. Our study might be one of the pioneering investigations delving into the application of HFNO in thoracic surgery patients during intubation. The aim is to compare the oxygenation status between the HFNO and mask preoxygenation groups, with the hypothesis that incorporating HFNO during DLT intubation can improve the lowest oxygen saturation levels.
Methods and analysis
Study design
The study was structured as a prospective, randomised, single-blind, parallel-controlled investigation. Figure 1 illustrates the study’s flow chart. The initial patient was enrolled in the study on 20 March 2023, and the study plan spans 2 years.
Flow chart of the research procedure. DLT, double-lumen tube.
Study sample calculation
The study aims to enrol 112 patients, determined based on a pretest estimation of the maximum time required for DLT intubation under FOB visualisation (5–6 min) and a minimum drop in SpO2 to (93.8%±7%) after anaesthesia induction with apnoea lasting more than 6 min, as reported in previous literature.17 The minimum SpO2 after implementing HFNO was estimated to be (97.3%±5.3%).18 Calculations were performed by using PASS V.15 software for the comparison of two independent sample means, with α=0.05 and β=0.2. This yielded a required sample size of 51 cases per group, accounting for a 10% drop-out rate, resulting in a sample size of 56 cases per group and a total of 112 cases across both groups.
Inclusion and exclusion criteria
Inclusion criteria
Participants meeting the following criteria will be included: age 18–60 years; patients scheduled for video-assisted lung surgery requiring DLT intubation; patients agreeing to participate in the study.
Exclusion criteria
Patients meeting any of the following criteria will be excluded: American Society of Anesthesiologists (ASA) classification>IV; patients with severe nasal obstruction; anticipated difficult intubation or difficulty with mask ventilation; morbid obesity (body mass index, BMI>35 kg/m2); abnormal airway anatomy; abnormal coagulation function; proposed emergency surgery; patients at high risk for reflux aspiration, including intestinal obstruction, full stomach and oesophageal reflux disease; maternal or lactating women.
Withdrawal criteria
All patients have the right to withdraw at any time. Participation can be terminated if the patient refuses to continue, withdraws consent, violates inclusion or exclusion criteria, or breaches the trial protocol. Reasons for withdrawal will be recorded on the CRFs. Patients who withdraw consent will be asked whether they are willing for data collected up to the point of withdrawal to continue to be used in the analysis. This ensures that as many patients as possible are included in our analyses on an intention-to-treat basis, even if some of their data are missing.
Randomisation and blinding
In this study, a randomisation method using randomly chosen block sizes will be employed.19 A professional statistician, uninvolved in data management, will use the Data Web data collection management system (https://www.empowerstats.net/dataweb2/) to create a central random sequence and allocate participants randomly into two groups at a 1:1 ratio. Stratification in our study will be based on preoperative pulmonary function test results, specifically using the Forced Expiratory Volume in one second (FEV1%) predicted values. Patients with an FEV1% predicted value below 80% will be deemed high risk for hypoxaemia. This criterion is based on findings from two key studies: one linking desaturation during FOB with low FEV1% predicted values and another demonstrating a similar correlation in elderly patients with Chronic Obstructive Pulmonary Disease (COPD) experiencing desaturation after a 6 min walk test.20 21 Random numbers will be placed in coded opaque envelopes and given to personnel not involved in anaesthesia and data collection for administration.
This study is structured as a single-blind trial, where patients and data collectors are unaware of the details. The intervention is carried out after the induction of anaesthesia, ensuring patient blinding. Although the operator, who cannot be blinded, performs the intervention, data collection and interviewing processes occur out of the operator’s view. Additionally, study operators and data collectors are unaware of each other’s collected information, maintaining a separation between the two roles.
Trial procedures
Patient screening
Patients and their representatives will be fully informed regarding the study and the subsequent follow-up procedures. We will enlist patients according to surgical schedule slated for elective video-assisted thoracic surgery of the lungs at Shenzhen Second People’s Hospital in China, primarily involving procedures such as lung wedge resection, lung segmentectomy and lobectomy, among others. Patients will be visited by investigators who are familiar with the protocol and duly authorised.
Induction of anaesthesia and DLT intubation
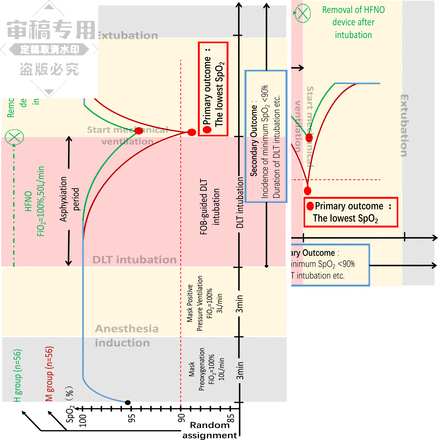
The same anaesthesiologist, with more than 3 years of anaesthesia experience, will execute the anaesthesia and DLT intubation protocol, as depicted in figure 2. All patients will observe a 12-hour fasting period and refrain from drinking for 4 hours prior to surgery. 30 min before entering the operating room, patients will receive an intravenous injection of glycopyrrolate (0.3–0.5 mg). On entering the operating room, patients’ vital signs, including ECG, non-invasive blood pressure, pulse oximetry, Bispectral index (BIS), and train-of-four (TOF) monitors, will be monitored. Subsequently, the anaesthesia machine breathing mask will be placed over the patient’s mouth and nose, and the oxygen flow rate will be set to 10 L/min. Preoxygenation will be conducted for 3 min at the normal tidal volume of natural breathing. Following this, target-controlled infusion (TCI) of propofol with a plasma concentration of 2.5–4 µg/mL and TCI of remifentanil with a plasma concentration of 1–3 ng/mL will be administered, alongside an intravenous injection of sufentanil at a dose of 0.2–0.3 µg/kg. Once the depth of anaesthesia monitor indicates a BIS value below 70, rocuronium (0.6 mg/kg) will be administered intravenously, and mask-assisted ventilation will commence. The fraction of inspiration O2 (FiO2) will be set at 100%, oxygen flow rate at 3 L/min, tidal volume at 200–300 mL and ventilation frequency at 14–18 breaths per minute.
Anaesthesia worksheet. Detailed layout of all steps on the interventional time scale. DLT, double-lumen tube; FOB, fibreoptic bronchoscope; HFNO, high-flow nasal oxygen; FiO2, fraction of inspiration O2; SpO2, pulse oxygen saturation.
In group M, once the TOF signals the disappearance of the T1 waveform and the end-tidal oxygen (EtO2) exceeds 90%, the vocal hilum is exposed using either direct laryngoscopy or video laryngoscopy. The DLT bronchial cuff is passed beneath the vocal hilum under direct vision. The catheter core is retracted, and the catheter is rotated by 90° before being advanced further until the main tracheal cuff passes through the vocal hilum, at which point advancement is halted. Subsequently, the FOB is inserted into the bronchial lumen of the DLT and slowly advanced down the trachea while maintaining proper orientation. On endoscopic identification of the carina, right and left bronchi, the FOB enters the corresponding main bronchus, guiding the DLT into the appropriate bronchial trunk. After confirming that the tip of the bronchial tube is clear and close to the secondary bronchial branch, the FOB is removed from the bronchial lumen, inserted into the tracheal lumen to verify correct bronchial cuff positioning, both cuffs are inflated, and the opening of the DLT tracheal lumen is confirmed to be unobstructed. Subsequently, the anaesthesia machine is connected to complete the intubation procedure. Double-lung mechanical ventilation is volume controlled, with parameters set at 6–10 mL/kg of ideal body weight for tidal volume and 12–14 breaths/min.
The HFNO device (Weishengkang Medical Technology, China) is connected to the power supply and oxygen source. The temperature is set to 34°C, oxygen concentration to 100% and flow rate to 50 L/min, with the device placed in standby mode. In the H group, patients receive mask-assisted ventilation after anaesthesia induction until the TOF indicates the disappearance of the T1 waveform and EtO2 exceeds 90%. The HFNO device, with a 4.5 mm inner diameter nasal cannula, is then worn and secured according to the preset pattern. The subsequent DLT intubation procedure mirrors that of group M. After connecting the DLT to the anaesthesia machine and initiating mechanical ventilation, the HFNO device is removed.
Anaesthesia maintenance
Intraoperative anaesthesia is sustained through a continuous infusion of propofol and remifentanil or a combination of 1%–3% sevoflurane, aiming to maintain BIS values within the range of 40–60. Non-steroidal anti-inflammatory drugs, such as flurbiprofen ester (50 mg) and the antiemetic ondansetron (4 mg), are administered intravenously before concluding the procedure. The ventilation strategy throughout the procedure adheres to a lung-protective approach. Before initiating OLV, FiO2 is set to 100% to facilitate the rapid collapse of the non-ventilated lung. During OLV, the FiO2 is gradually reduced to the minimum value (40%–100%) necessary to sustain SpO2 above 90%–92%.
Evaluation and follow-up
Following surgery, the patient is transferred to the postanaesthesia care unit (PACU), and the postoperative analgesic protocol is determined based on the nature of the procedure. Follow-up concludes on the patient’s discharge from the hospital. Data collection and follow-up on all patients will be conducted by researchers who are unaware of the study intervention.
Outcome measures
Primary outcome
The primary outcome indicator is the minimum SpO2 measured through capillary oximetry during DLT intubation.
Secondary outcome
Apnoeic oxygenation outcome indicators:
Incidence of minimum SpO2<90% during DLT intubation.
Incidence of minimum SpO2<95% during DLT intubation;.
Duration of DLT intubation.
The initial end-tidal CO2 measurement taken immediately after intubation.
The initial EtO2 measurement taken immediately after intubation.
DLT intubation-related indicators
Incidence of difficult airway (defined as failure of visual laryngoscopic intubation and conversion to FOB-guided intubation).
Incidence of bronchial misalignment involving DLTs.
Complications during intubation: regurgitant misaspiration, laryngospasm or bronchospasm, tracheal or bronchial laceration, air pressure injury, mean arterial pressure <55 mm Hg or initiation of vasoactive drugs, systolic blood pressure >180 mm Hg, severe arrhythmia, incidence of lip and tooth injury.
Operator satisfaction with the intubation process is recorded immediately after intubation. It is assessed on a scale of 0–10, with 0 indicating extreme dissatisfaction and 10 indicating extreme satisfaction.
Prognostic indicators of surgery:
Incidence of postoperative airway-related complications (postoperative sore throat, postoperative hoarseness and nasopharyngeal cavity dryness).
Incidence of postoperative nausea and vomiting.
Incidence of low oxygen saturation (SpO2<90%) in the PACU.
Incidence of postoperative pulmonary complications, including atelectasis, pulmonary infection, pleural effusion, postoperative bleeding, bronchopleural fistula and pulmonary embolism, is recorded prior to patient discharge.
Patient satisfaction with anaesthesia, recorded on the first day after surgery on a scale of 0–10, with 0 indicating extreme dissatisfaction and 10 indicating extreme satisfaction.
Length of postoperative hospital stay.
Adverse events
Adverse events encompass all undesirable or unintended illnesses and signs that may potentially be linked to the investigational drugs. Continuous monitoring of all adverse events associated with this trial will occur until their resolution, stability, or confirmation of no relation to the trial. In the event of adverse occurrences, immediate reporting to the research department and the principal investigator will take place to assess the severity and consequences of the injury. All adverse events related to this study will be documented and reported to the ethics committee within 1 week. The principal investigator will bear full responsibility for the reporting of all adverse events.
Data collection
To ensure data accuracy, all intubations will be meticulously recorded on video. A dedicated camera will be strategically positioned to provide an unobstructed view of the entire procedure, including the pulse oximeter displaying oxygen saturation levels. The primary outcome, considered of utmost importance, will undergo rigorous scrutiny. An independent assessor will comprehensively review the procedure via the video recordings to confirm the primary outcome. In the rare event that the video cannot be used for verification, the primary outcome determination will rely on the maintained CRF.
In this study, a combination of paper-based data collection and electronic database recording was employed for data management. Initially, research personnel transcribed data from subjects' original observation records onto paper CRFs. These CRFs were then signed by the principal investigator and promptly submitted to the clinical trial data custodian. Data input and management were handled by a team separate from the research intervention, using an electronic database system (data web, https://www.empowerstats.net/dataweb2/) for data entry and validation. The hospital’s fund committee will convene regular meetings to oversee project progress. This process will remain independent of investigators and the sponsor. Data collection will adhere to a specific schedule as depicted in table 1.
Study schedule
Statistical analysis
Analysis set
For both primary and secondary endpoints, the intention-to-treat analysis will encompass all randomised patients, excluding only those who withdrew consent. Patients will be analysed according to their assigned groups, irrespective of the treatment received. Additionally, a per-protocol analysis will be conducted as a sensitivity analysis, excluding patients who deviated from their assigned treatment. Safety analyses will include all treated patients with at least one follow-up visit.
Baseline analysis
Mean and SD will be used for normally distributed outcomes, while median and IQR will be applied for skewed outcomes. Categorical variables will be summarised with frequency counts and percentages as appropriate. Independent t-tests or Mann-Whitney tests will compare continuous variables, and a χ2 test will assess categorical variables.
Primary outcome analysis
Given the skewed distribution of the lowest SpO2, the Mann-Whitney U test will assess group differences for the primary outcome. Additionally, rank analysis of covariance (Quade’s test) adjusted for age, sex and other relevant disease-related characteristics will be applied.
Secondary outcome analysis
Comparison of secondary outcomes between groups will employ a χ2 test for differences in proportions. Student’s t-test and Mann-Whitney U test (as appropriate) will analyse parametric and nonparametric data, respectively. Time-to-event endpoints will be analysed using the log-rank test to compare the time from randomisation to endpoints, and a Cox proportional-hazards model will estimateHRs and 95% CIs.
Missing data
For outcomes with a substantial amount of missing data (≥5% for a specific outcome), a multiple imputation model will be employed to handle missing data.
Subgroup analyses
Subgroup analyses for the primary outcome will be conducted based on gender, BMI, age, preoperative FEV1 expected value, DLT intubation time and the presence of a difficult airway.
Software and significance threshold
A significance threshold for p values will be set at 0.05 for all statistical tests. Given the potential for type I error due to multiple comparisons, results from analyses of secondary endpoints should be interpreted as exploratory. The statistical analyses will be performed using R (V.4.2.0).
Patient and public involvement
No patients or the public were involved in the design, conduct, reporting and dissemination plans of this research.
Discussion
The mechanism underlying the oxygenation provided by HFNO remains a subject of ongoing research. The current study suggests several potential mechanisms: HFNO delivers a consistent inhaled oxygen concentration, establishing an upper airway-alveolar-pulmonary capillary oxygen partial pressure gradient. Additionally, its production of low-level continuous positive airway pressure (2.7–7.4 cm H2O) facilitates clearance of nasopharyngeal dead space, reduces nasopharyngeal resistance, promotes alveolar recruitment, decreases respiratory work and prevents the development of pulmonary atelectasis and bronchospasm. Cardiogenic oscillations, involving changes in airflow caused by cardiac contraction, may contribute to gas exchange during apnoea induced by HFNO.8 22–24
This study represents the first attempt to use HFNO in thoracic surgery patients during the intubation period. Given the extended duration of apnoea during fibreoptic-guided DLT intubation and the large size of the double-lumen catheter, demonstrating that HFNO-assisted apnoeic oxygenation can extend the safety window would provide evidence that high-flow oxygen can traverse the double-lumen catheter and the narrow tracheal gap. This offers practical insights into the mechanism of HFNO oxygenation.
We employ block randomisation with random block sizes to mitigate systematic biases to a certain extent. All procedures in the operating room will be executed by the same anaesthesiologist, thereby preventing bias arising from variations in skills and experience among different anaesthesiologists. The operating room procedures will be documented using cameras to optimise data recording accuracy. Our selection of the lowest SpO2 as the primary outcome, rather than the incidence of deoxygenation, is grounded in the belief that, unlike the relatively subjective nature of deoxygenation incidence, the lowest SpO2 during intubation is an objective and quantifiable measure directly reflecting the patient’s oxygenation level. This choice enhances the accuracy of evaluating the relative effectiveness of HFNO and mask oxygenation during intubation, thereby bolstering the internal validity of the study. Aligned with prior Intensive Care Unit (ICU) studies18 25 that also designated the lowest SpO2 as a primary endpoint, our study conforms to established literature for meaningful comparisons and references.
HFNO is now widely employed to manage acute respiratory failure.26–28 Various study findings indicate the benefits of preoxygenation with HFNO before endotracheal intubation.11 29 30 The potential for using HFNO during invasive operations to enhance safety is being demonstrated.31 32 The application of HFNO for oxygenation in challenging tracheal intubation situations is an emerging area of research,14 15 encompassing contexts such as obesity, infants and children, and ICU settings,18 33 34 demonstrating commendable efficacy in oxygenation. In the current study, DLT intubation was performed using direct fibreoptic-guided methods, aiming to reduce the incidence of bronchial misalignment. However, this approach may lead to prolonged intubation duration and periods of hypoxia, potentially resulting in adverse effects. To investigate whether the supplemental use of HFNO during this period of asphyxiation could enhance hypoxia-related indices and minimise intubation-associated complications, as well as perioperative-related complications, was explored.
This study has several limitations. First, it is a single-centre study, and all tracheal intubation procedures will be performed by the same anaesthesiologist, which may limit the generalisability of the results. However, we will precisely record the DLT intubation time for each patient. This will indirectly reflect the impact of intubation difficulty levels on both groups, further ensuring the consistency and reliability of our study results. Second, due to ethical considerations, we did not include patients at higher risk of hypoxaemia during the DLT intubation procedure (such as obese and elderly patients), although they may potentially benefit from HFNO. However, we plan to conduct subgroup analyses after completing data collection to investigate whether high-risk populations derive greater benefits. The results of this study will lay the groundwork for future research on the use of HFNO during DLT intubation procedures in these specific populations.
In conclusion, our randomised, controlled, single-blind study aims to validate that HFNO constitutes a novel approach for enhancing oxygenation during DLT intubation, consequently offering safer intubation conditions.
Ethics and dissemination
Ethics approval and consent to participate
The study protocol has secured approval from the Ethics Committee of Shenzhen Second People’s Hospital (20220412005-FS01) and is registered with ClinicalTrials.gov (ID: NCT05666908). This protocol is version 2.0, dated 17 December 2022. Clinical trials will adhere to the Declaration of Helsinki and pertinent Chinese regulations for clinical trial management. The initiation of the trial is contingent upon approval by the Clinical Research Ethics Committee.
The research physician is obligated to furnish each patient with a comprehensive written explanation outlining the study’s purpose, procedures and potential risks before their enrolment. Patients must be apprised of their right to withdraw from the study at any point. A written informed consent form must be provided to each patient before enrolment. The research physician is responsible for obtaining informed consent before each patient’s participation, and the consent forms will be retained as part of the clinical trial documentation. In this trial, patients and their authorised representatives, in an anaesthetised state, should be informed of the aforementioned details before surgery and sign the informed consent form and a power of attorney (using the standard version from our institution) as evidence.
Confidentiality
The study results will be disseminated in the form of a scientific paper, with the imperative that all personal information of the participants remains confidential. In adherence to Good Clinical Practice requirements, Electronic Data Capture data must be preserved for a minimum of 5 years.
Ethics statements
Patient consent for publication
Acknowledgments
The authors express gratitude for the assistance provided by colleagues at the Department of Anesthesiology, Shenzhen Second People’s Hospital.
References
Footnotes
Contributors Ren He and Nanbo Luo contributed to the study design, methodology, investigation, data curation, formal analysis, and original draft writing. Da Yao and Yonghan Jiang followed the research, contributed to data curation and validation. Weijun Zheng was responsible for data curation and data analysis. Zhi Li and Yuxiang Fang participated in the critical review and revision of the manuscript. Nanbo Luo and Zhiheng Liu were in charge of project administration, resource management, and supervision. All authors have read and approved the final manuscript.
Funding This study received funding from the Shenzhen Second People’s Hospital Clinical Research Fund of Guangdong Province High-level Hospital Construction Project (grant numbers 20223357001, 20223357006).
Disclaimer The funding played no role in the study’s design, the collection, analysis, and interpretation of data, or in writing the manuscript.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.