Article Text
Abstract
Objectives Public access databases such as clinicaltrials.gov achieve dissemination of clinical trial design and aggregated study results. However, return of participant-level data is rarely done. A key barrier includes the proprietary ownership of data by the sponsor. Additionally, investigators may not have access to centralised data, and per International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice, must maintain the confidentiality of participants. This study piloted an approach to return both individual and aggregate clinical trial data to parents of children participating in a series of open-label clinical trials.
Setting and design A small biotech company obtained central ethics approval (centralised institutional review board [IRB], non-exempt). The study was advertised via parent advocacy groups. Parents of trial participants were offered the option to contact an employee (coordinator) within the company, requesting return of their child’s study results. Ethics approval covered participation in six countries. The study focused on the sequential clinical trials of vamorolone VBP15-002 (NCT02760264) and VBP15-003 (NCT02760277) (post-results).
Interventions Contact initiated by the parent enabled the coordinator to obtain informed consent (and separate General Data Protection Regulations consent), with phone translation when needed. Using date of birth and study site location provided by the parent, the data manager reported the participant number to the coordinator. The coordinator retrieved and compiled data, along with an aggregate summary, which was mailed via a password protected and encrypted memory device to the parent. Prereturn and postreturn surveys were sent to consented parents (n=19; 40% of 48 total trial participants) and investigators.
Results Prereturn surveys indicated a request for as much data as offered, in all formats offered. Postreturn survey showed high satisfaction with the process and data returned. Survey of the physician site investigators (n=10; 100% participation of investigators) voiced general satisfaction with the process, with some reservations.
Conclusions This pilot study demonstrates an innovative, cost-effective, centralised and labour conservative approach to return of participant-level and aggregate data to participants in studies.
- ETHICS (see Medical Ethics)
- Health Literacy
- Patient Satisfaction
- Clinical Trial
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A strength is the novel approach of return of patient-level clinical trial data to trial participants and their families, directly by the trial sponsor.
A strength is the survey of the parents of the trial participants regarding clinical trial data they wished to have returned, the format of these data and their satisfaction with the process.
A strength is the survey of physician attitudes regarding the direct communication of the sponsor and trial participants.
A limitation is the small number of trial participants (n=19) and physicians (n=10) that participated in this pilot study.
Introduction
Health authorities, academic societies and patient advocacy groups are increasingly focused on increasing transparency of clinical trial design and conduct, as well as data sharing and data stewardship. This is reflected in the US 21st Century Cures legislation which supports the National Institutes of Health data sharing mandates1 2 and is further exemplified by recent European Union Clinical Trial Regulations, which note key initiatives of improving information sharing and increasing transparency of information related to clinical trials (https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials/clinical-trials-regulation). Access to participant-level data enable alternative approaches to data analysis, including meta-analyses and modelling to facilitate drug development (eg, predictive clinical disease progression models, clinical trial simulation tools).3 Data siloes, driven by economic and academic incentives, have the potential to undermine development of treatments for rare diseases.4 Studies demonstrate that most clinical trial participants view data sharing positively, despite some concerns related to confidentiality and data security, awareness about access and control, and potential harms resulting from these risks.5 6
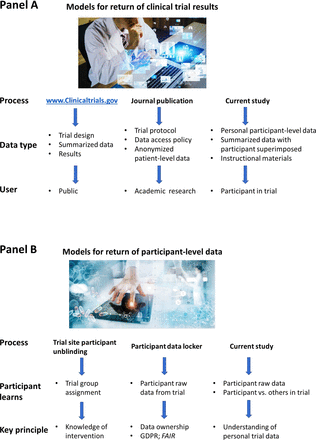
Clinical trial data disclosure or sharing may take several forms, including the posting of aggregate results on a public or private website, sharing of deidentified data with a third party (for research or other purposes), or return of an individual’s personal health data back to them (figure 1A). Some data collected during a clinical trial are monitored in order to assess a person’s well-being during the trial, or response to therapy (eg, weight, height, clinical chemistries); some of these data could duplicate data found in their medical record or be used by their physician during their clinical care. Other data collected during a trial may be less relevant to their healthcare (eg, biomarkers and changes in outcome measures that were selected to measure the effect of a drug); often these data are not regularly assessed during the care of a patient. Sometimes these data are not accessible to their physician during the trial due to use of a central laboratory or a non-CLIA-approved laboratory, and even if they are, may not be easily interpreted by the physician because they are exploratory, or intended to assess the pharmacodynamics of a drug. Participants (or parents) may misunderstand that biospecimens are being collected for research purposes only, and not for their direct care.
Models or return of clinical trial results and return of patient-level data. Panel A: models of return of clinical trial results. Panel B: models for return of participant-level data. Photographs licensed from iStock.
With the emergence of the General Data Protection Regulations (GDPR) in Europe, there is an acknowledgement that individuals have a fundamental right to ownership of their own personal health data, including data collected during a clinical trial (figure 1B).7 Efforts are underway to enable individual ownership of personal health data through secure ‘data lockers’, and FAIR consensus foundational principles have evolved to create a construct for such data return, ownership and sharing (Findability, Accessibility, Interoperability, Reusability).8 Patient advocacy groups have begun to focus on mechanisms to encourage and implement FAIR data lockers for their stakeholders.9 We hypothesised that the driving principle for a clinical trial participant may be ‘a right to know and understand’ their personal clinical trial results, and not as much a ‘right to own’ their clinical trial data. Additionally, while ‘machine operability’ is imperative for data sharing under GDPR, a recent study of clinical trial participants demonstrated a preference for receiving data by mail and not via a website.10
We sought to understand parent/caregiver and physician views on return of their child’s individual personal health data at the end of an open-label clinical trial. We also sought to develop a cost-effective process for returning clinical trial data directly to participant families, while viewing it as an opportunity to be transparent about how these data were similar or different from data obtained by their physician during clinical care. The focus of our study was clinical trials of young boys living with Duchenne muscular dystrophy. This genetic disorder has a worldwide incidence of 1:5,000 males worldwide, and shows clinical onset around 5 years of age, and progressive weakness and disability. The clinical trials were supported by public funds (National Institutes of Health (USA), and European Commission Horizons 2020 (EU)), and were testing vamorolone, a disease-modifying therapy intended to be a safer alternative to corticosteroid standard of care. Vamorolone has received regulatory approval from FDA (USA; 2023), EMA (EU; 2023) and MHRA (UK; 2024) based in part on these clinical trials.
In 2019, the sponsor of the trials, ReveraGen BioPharma, received an Administrative Supplement for Research on Bioethical Issues award from the National Institutes of Health (‘Establishing a Cost-effective Return of Results to Parents of Boys in VISION-DMD Clinical Trials’). The goal of this study was to pilot a centralised approach for return of participant-level data to families participating in clinical trials of vamorolone. Here, we discuss this pilot process using data from a series of small open-label trials and present findings from parental and physician surveys, intended to inform application of this process to other studies.
Methods
Patient population and trial design
This study was focused on participants in two vamorolone trials, VBP15-002 (4-week dose-ranging study; NCT02760264),11 and VBP15-003 (24-week extension study; NCT02760277).12 These two trials were sequential open-label trials, with 48 participants with Duchenne muscular dystrophy (DMD), age 4 to <7 years at study entry. VBP15-002 was a multiple-ascending dose study over a 24-fold range of vamorolone doses (0.25 mg/kg/day to 6.0 mg/kg/day), recruited 12 participants in each of 4 dose groups, and was a 4-week safety and pharmacokinetics study (2 weeks on drug, 2 weeks washout). All participants were then enrolled into a 24-week dose-finding study at the same doses (VBP15-003), with motor outcomes at baseline, 12-week and 24-week treatment, and laboratory outcomes (safety labs, exploratory biomarkers). In this report, we focused on test results reported back to patient families. These included the motor outcomes Time to Stand from Supine velocity (in event/s), 6-min walk test (in metres walked), time to run/walk 10 m (in m/s), time to climb 4 stairs (in event/s) and NorthStar Ambulatory Assessment (total score). Blood laboratory tests (safety biomarkers) assessed in a central laboratory included creatine kinase, osteocalcin, N-terminal propeptide of type 1 collagen (P1NP), C-terminal telopeptide of type I collagen (CTX1), morning cortisol, fasting insulin and glucose, and glutamate dehydrogenase. Exploratory blood pharmacodynamic protein biomarkers, tested at Somalogic, were CD23, MDC/CCL22, IL22BP, lymphotoxin a1b2, IGFBP2 and MMP12.
Patient and public involvement statement
The concept of this study evolved from discussions with parents of patients and advocates at disease-focused conferences. Multiple patient advocacy group leaders, physicians and parents of children with DMD were consulted about the concept of this project, and were asked to comment on and contribute to the design of the data return and questionnaire content.
Consent of participants
Western IRB (recently renamed WCG; https://www.wcgclinical.com/about/) is an accredited ‘central’ ethics review panel (not affiliated with a single institution). Clinical trials funded by the US National Institutes of Health now require such centralised ethics review. The approval included advertisement of the study via patient advocacy groups in countries in which enrolment had taken place (USA, Canada, United Kingdom, Sweden, Israel and Australia), and the ability to consent the participant via telephone with use of a telephone interpreter if requested by the parent (figure 2A). The advertisements included the contact information of a single coordinator employed by the sponsor; a strict firewall was established where the coordinator shared no identifying information with any other employee of the sponsor or others.
Return of results design. Panel A: overall study design of sponsor direct return of participant-level and aggregate data to clinical trial participants. Panel B: example of graphical return of participant-level data, showing the participant’s data relative to other participants in the same treatment group.
Once a trial participant family (parent) contacted the coordinator and requested participation in the return of results study, the coordinator then explained the study and conducted the informed consent process by teleconference. The informed consent was sent via Adobe Acrobat Sign for signature (online supplemental file 1). For patients in European countries, a separate GDPR consent was also completed, and signed via Adobe sign (online supplemental file 2). Only those who signed informed consent participated in the return of results study (figure 2A). Following completion of informed consent, the coordinator collected the following information from the family and stored it in a password-protected, cloud-based file: parent’s name, home address, parent’s email address, child’s study site and child’s date of birth. The child’s study site and date of birth were provided to the data manager, who identified the study subject number. The data were extracted from the electronic data capture system using only the subject number, and then were presented in a standardised format and converted to a pdf file.
Supplemental material
Supplemental material
Return of clinical trial results to families was done by sending (by mail) an encrypted and password-protected USB memory device. The memory device used SanDisk Secure Access software (128-bit AES encryption to create a password-protected folder—SanDiskSecureAccess Vault—on the flash drive). Locked files were moved into the SanDiskSecureAccess Vault and only accessed with a password sent separately via email to the family.
Surveys
Three surveys, two for parents, and one for their physicians, were developed, and feedback was sought on draft content of surveys from parents, stakeholder foundations and physicians prior to finalisation and dissemination.
The first parental survey was administered after signing of consent to participate in the study, but before results were returned (online supplemental file 3). This parental survey was designed to instruct parents on the types of data available from clinical trials (motor outcome, clinical laboratory, exploratory biomarkers), and ask what type of data they were interested in receiving (aggregate, patient level), and in what data format for data return. The second parental survey was administered after the return of results, to gauge parental satisfaction with the materials received (online supplemental file 4).
Supplemental material
Supplemental material
A third survey was developed to administer to the clinical trial site physicians caring for the patient and patient family that had consented to participate in the return of results (online supplemental file 5). The purpose of this survey was to assess the opinions of the physicians regarding the return of patient-level clinical and laboratory data directly from the sponsor to the parents. The physicians responsible for the participants during the trial also followed the patient for the subsequent 2 years, as all participants enrolled in a 2-year long-term extension study. Thus, the same physician cared for the participant during the trial, and afterwards during the return of results and associated surveys.
Supplemental material
Results
Parental attitudes and desires regarding clinical trial return of results
Of the 48 patient families participating in the VBP15-002/003 clinical trial of vamorolone, 19 (40%) responded to advertisements via stakeholder foundations (58% North America (US, Canada), 42% Europe and Israel). We also developed an informational sheet that could be handed out at the clinical trial sites during patient family follow-up visits, but clinical trial sites were uncomfortable handing out this informational sheet without their own institutional ethics approval.
The full results of the survey of 19 parents prior to return of results are provided (online supplemental file 6). We queried whether aggregate or individual participant-level data were important to parents, and the majority (90%) felt that access to both types of data was ‘very important’. We then asked if data should be best presented in tabular, or graphical form. Most parents (97%) indicated that receipt of data in both formats was preferred. We then queried what biomarkers were important to report back to parents, giving examples of safety labs (cortisol, insulin, glucose), bone turnover biomarkers (osteocalcin, P1NP, CTX1) and exploratory efficacy biomarkers. The majority of parents responded that they would like all data reported to them.
Supplemental material
For the questions, “What do you expect you would do with the information returned that summarizes results for all boys in the trial?’ most responses acknowledged that the return of data would be for informational purposes only. For ‘What do you expect you would do with information return on your son’s individual results?’ most again responded that it would be for informational uses only, although 4 (of 18) mentioned the possibility of discussing the data with their physician.
Return of results
Both aggregate and individual (participant-level) were returned to patient parents on a password-protected USB memory device sent via the mail. An example report is provided (online supplemental file 7). The report included a 2-page educational introduction to aid interpretation of the report. This included definitions of efficacy and safety outcomes, the concept of aggregated data for interpretation of drug efficacy and safety and distinctions between data generated in a research study versus clinical care. For educational purposes, the report also elaborated on challenges facing sponsors in terms of return of data, including confidentiality firewalls and risk for parent/patient overinterpretation of research data regarding clinical care. The following 15 pages provided the trial participants individual clinical trial data (motor outcomes, quantitative muscle testing, anthropomorphic data and laboratory data), as well as his data superimposed on aggregated data, both as tabular and graphical form for key clinic visits (baseline, 12 weeks and 24 weeks treatment). The graphical form of data presentation showed each individual in the specific vamorolone dose group (n=12), with their child’s data colour coded within this group (figure 2B).
Supplemental material
Parent follow-up survey
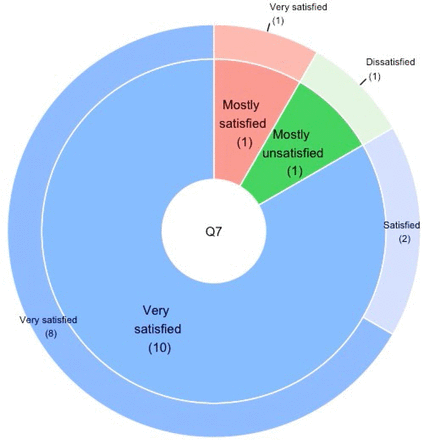
Of the 19 families to whom the prereturn survey was completed and results were returned, 12 of these completed the postreturn survey (63%). The complete responses are provided (online supplemental file 8). The majority of the families were ‘very satisfied’ with both the return of data approach (10/12; 83%), and method of return of data on a password-protected USB memory device (8/12; 67%) (figure 3). One family expressed dissatisfaction with both of these queries (1/12; 8%), but did not provide reasons for their dissatisfaction. Most families (18/19) had no technical issues with receiving the materials on a password-protected USB; one family had technical problems and was mailed a hardcopy of the materials.
Supplemental material
Post return of results parental satisfaction. Inner pie: parental satisfaction with return of data approach used by the sponsor. Outer donut: parental satisfaction with delivery of the data by mailed, encrypted memory stick.
When asked if they felt that the return of results was important to them, all (12/12) replied that it was ‘very important’ (7/12; 58%) or ‘important’ (5/12; 42%). When given an open-field query for why they felt the data return was important, 10 responded (see table 1). The responses primarily oriented about the importance of knowledge about the trial and being informed about the child’s health.
Responses of parents of participating children in the clinical trial when asked why they thought that data return was important to them, and their physicians regarding their degree of support of sponsor direct return of data to families
Most parents indicated that it was important to see their child’s data in comparison to others in the trial (11/12; 92%) and provided free text justifications that were concordant with increased information exchange which is preferred over more narrow information regarding their child. Parents were queried regarding the amount of data provided, and the majority (11/12; 92%) responded that it was ‘about the right amount of information’, and 1 parent reporting that it was too little information.
Parents were asked if they would have preferred their child’s data returned to them via their physician, rather than the sponsor (ReveraGen). Most (8/12; 67%) responded “I’m neutral; either way would be fine”; some responded that they would strongly prefer to receive their child’s data from the sponsor and not their physician (3/12; 25%), and a single parent stated that they mostly agree with their preference for receiving the data from their physician, but not strongly (8%). When the respondents were stratified by North America versus Europe, there were no differences.
The parents were queried as to whether they had shared the returned data with others. Half of respondents had shared data with family members, 42% with healthcare providers, 17% with friends and 8% with teachers; 42% responded that they had not shared the data with anyone. When asked if they would participate in such a return of results study again, all responded affirmatively (12/12). Asked if they had regret regarding participation in this study, all responded that they did not have regret.
Survey of clinical trial site physicians
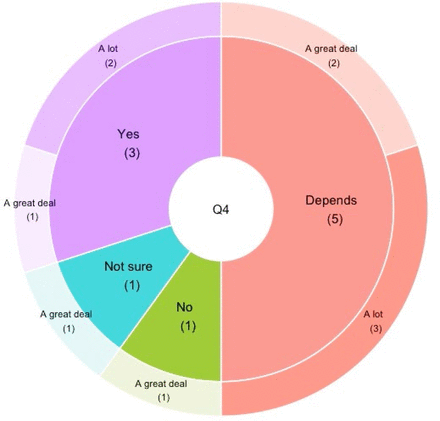
Of the 10 physicians that we asked to complete the survey (eg, those physicians following the 19 patients), all 10 responded. The trial had 12 sites in 6 countries, so this represented 83% of physicians and sites. The complete responses are provided (online supplemental file 9). The physicians were unanimous in their opinion that parents put a great deal of importance on receiving both individual and aggregated trial data, and all physicians affirmed that families should receive these data if requested by the family (figure 4). We asked, “Do you agree with the concept of a Sponsor returning individual clinical trial data directly to trial participants?” most (8/10) were supportive of this, but 5 of these 8 expressed some reservations (‘Yes, but it depends on the circumstances’); 1 was not sure, and 1 responded ‘no’. When respondents were stratified by North America versus Europe, North American physicians (n=6) voiced more enthusiasm for this approach, whereas the European physicians (n=3) were less enthusiastic (online supplemental file 10). When asked to elaborate on any concerns of a sponsor returning participant-level data directly to families, responses are shown (table 1).
Supplemental material
Supplemental material
Physician attitudes towards returning clinical trial data to participating families. Inner pie: physician agreement with concept of sponsor returning individual data directly to participants. Outer donut: physician perception of importance families place on receiving individual trial results.
Cost-effectiveness analysis
The clinical trials that were the focus of this study were managed via a public–private partnership model, with funding to the for-profit sponsor (ReveraGen) from the National Institutes of Health and European Commission. The sponsor contracted with each academic clinical trial site directly (11 sites in 6 countries) and thus had access to all costs associated with contracting of the academic clinical trial sites, ethics review and participant visits to the site. We estimated costs of the following four models of returning participant-level clinical trial results to clinical trial participants:
Current model. Central ethics review held by the sponsor, and direct communication with clinical trial participant families.
Model 2. A stand-alone study, with new contracts for return of results between the sponsor and the participating academic clinical trial centres, inclusive of clinical trial site ethics review, and on-site visit of the participating family for in-person return of participant-level data.
Model 3. Similar to model 2, but with remote (teleconference) delivery of the participant-level clinical trial data by the academic clinical trial site to the study participant. This model does not include patient travel-stay costs to go to the academic clinical trial site.
Model 4. In this model, the return of results is included in the clinical trial protocol from initiation of the contracts with each academic clinical trial centre. In this model, the initial costs of the ethics review are covered by the costs for the clinical trial protocol. However, the site would need to remain open (active contract) for about 2 additional years beyond the typical close-out (the clinical trial would need to be completed, data unblinded and then the return of results initiated). This model assumes in-person delivery of the results to the study participant by the academic clinical trial staff.
The results of this financial impact analysis are shown (table 2). The realised costs associated with data management and reporting (extraction of individual participant data, assembly into participant-specific reports, reporting) were US$78 585, and this was assumed to be a fixed cost across all models. In the current model, the focus of this manuscript, there was only the additional incremental cost of a centralised ethics approval held by the sponsor, for a total cost of US$86 171. The alternative models where participant-level data was returned to participants by the clinical trial sites following those participants were considerably more expensive, with costs driven by the ethics review that would be required at each of the 11 participating sites, the time and effort of clinical site staff, institutional overhead costs associated with site contracts, and (models 2 and 4) the cost of participant travel to and stay near the clinical trial site for in-person return of clinical trial data.
Real or predicted costs associated with different return of participant-level data to clinical trial participants
Discussion
We carried out a centralised return of both participant-level and aggregated clinical trial data to parents of children in an open-label dose-ranging study of vamorolone. Key to our approach was the efficient navigation of human subjects oversight, where we received a single-centralised ethical approval for patients worldwide to contact the sponsor to request the clinical trial data on their child. Our method of alerting patient families of this return of results project was through stakeholder foundations in the six countries in which the clinical trial was being conducted (US, UK, Canada, Israel, Australia and Sweden). As the parents were contacting the sponsor directly to request information on their own child, the ethical committee felt that it was adequate to remotely consent parents (with a translator if needed), and that the study was ‘expedited with no requirement for continuing review,” much as other survey-type research projects.
The more typical alternative approach of returning clinical trial data to participants is through collaborating clinical trial sites via their healthcare providers. This would require (in our case) local clinical site ethics approval (12 sites in 6 countries), as well as contracts between the sponsor and each site to carry out the return of results. Our approach of implementing direct contact between the parents contacting the sponsor greatly simplified the otherwise complex challenge of returning patient-level clinical trial data to clinical trial participants. Critical to our approach is that the parents initiate contact with the sponsor, not the sponsor with parents. Also central to our approach is a ‘data/information firewall’ within the sponsor, where only a single employee had direct contact with families, and no deidentifying information was relayed to any other employee of the sponsor. Additionally, an interpreter in the parents’ native language was always made available, and consent forms were translated to the parents’ native language.
We queried the attitudes of participating parents both before the return of results, to learn what type of information they felt was important, and how they would like these data to be provided to them. In general, parents expressed a strong desire for as much information as possible, in all formats offered (individual, aggregate; tabular, graphical). Thus, tailoring of information provided to the families was not needed; all families expressed a desire for all information offered. In returning the data to participants’ parents, we instructed that this was clinical research data and not generally relevant to the clinical care of their child. Also, we provided tutorials on motor outcome measures, and interpretation of clinical laboratory and exploratory biomarker data. Participant families who participated in the return of results directly by the sponsor expressed overall satisfaction with all aspects, including the process, the amount of information received, the graphical and tabular presentation, the presentation of both individual and aggregate data, and the manner in which it was received (password protected and encrypted USB memory stick mailed directly to the family). We note that our approach included two-factor authentication (direct mail, separate password communication), which is important to maintain privacy and confidentiality.
We found that, of the sample of parents who requested their child’s data, most would prefer to obtain the data directly from the sponsor, or were indifferent to whether they obtained data from the sponsor or their physician. None of the parents indicated a strong preference for obtaining the clinical trial data from their physician. This finding supports our approach to providing individual-level data directly from the sponsor. All participants felt that return of data was quite important to them, and parents showed a variable degree of sharing of information with family, friends, teachers and their physicians.
Physician respondents unanimously acknowledged the importance that families place on return of clinical trial data. Some had reservations about return of results without involving clinicians or the clinical site investigators. When physician responses were stratified by North America versus Europe, North American physicians were more accepting of the direct return of data participants by the sponsor (p=0.065; Wilcoxon rank-sum test), although numbers were small and difference not significant (North American physicians n=6; European n=3). These concerns, and potential cultural differences in acceptance by physicians, will need to be further explored and addressed in future return of results approaches.
For parents of children with DMD, participation in clinical research is a balance of hope and expectations. Parents of children with DMD report a feeling of investment in the trial.13 In one study, at the termination of a trial in DMD, parents wished for more communication from the sponsor. Some parents felt that when the trial ended, the partnership between the parent and sponsor ‘broke down’ and that the sponsor no longer valued them.14 Parents describe the significant burdens that participation in clinical trials places on their families.15
In keeping with the ethical principles of beneficence and autonomy, return of data demonstrates respect for participants’ ownership of their health data, encourages family engagement and fosters increased trust of researchers by patients who are clinical trial participants and their families. Operationally, there is a disconnect, as the clinical trial site personnel and physician have direct contact and responsibility for care for the patient, but typically do not have access to all of the patient’s data. Direct industry–patient interaction for returning individual results after trial completion, without the study site/physician interface, has not been common historically due to potential for perceived loss of patient confidentiality, concerns about results interpretation and the potential for clinical follow-up for actionable findings if clinicians are not involved, and possible conflict of interest. However, our approach demonstrates that this can be achieved by having an internal coordinator who is not involved in the study conduct, keeps records confidential and is under a ‘firewall’ of confidentiality when it comes to the study. Another approach could be to use a third party vendor, though this would increase costs and complexity. Sponsors may perceive the return of results to trial participants as a risk to the participant and the trial, or at least as a distraction to the sponsor, adding additional time and cost to the drug development process. We have demonstrated that this can be a relatively straightforward process that is not costly and can be done after study completion, and public disclosure of trial data. Alternative models of return of results require contracts between the sponsor and the participating clinical trial sites, and this adds considerably to the costs and administrative complexity (table 2).
While the current proof-of-concept study is admittedly quite small, we envision that such return of results could be scaled up without additional barriers. Assuming a large multinational phase 3 trial, either the sponsor or a contract research organisation would receive central ethics approval for return of results to trial participants requesting the data (as we have done here). This participant-initiated request would permit deidentification of the subject in the data, and direct return of the data to the participant. The sponsor could either do this internally with appropriate GDPR firewall (as we have done), or could contract a third party to carry out the process at arm’s length.
Not all clinical trial data are relevant to a patient’s medical care, and indeed may not add value or be acceptable to add to the participant’s electronic medical health record. While clinical trial data are personal health data, it likely has different value to a clinical trial participant compared with their own electronic medical health record. The National Academies of Science (NAS), Engineering, and Medicine convened a committee that published ‘Returning Individual Research Results to Participants: Guidance for a New Research Paradigm’, a process-oriented approach to return of results that considers value to the participants, feasibility of return and quality of research results.16 The NAS committee formulated six principles to help guide deliberations and development of recommendations presented in their report. One principle was that the potential value of returning individual research results must be carefully considered along with the trade-offs for research participants, investigators, research institutions and society. According to the committee, ‘value’ should consider the perspective of the participant (or parent) and might entail clinical utility or personal utility, as well as personal meaning. Thus, the value of a result is not necessarily tied to its use, as viewed solely through the eyes of the clinician or sponsor. DMD parents and advocacy groups in the USA and European Union clearly indicate that they value provision of individual and aggregate clinical trial results to the study participant.
Recent reviews of efforts to return clinical trial data to participants have found that these are relatively rare and typically only include summarised or aggregate results (not personal participant-level data). Bruhn et al17 studied clinical trials in a period from January 2008 to August 2019 and identified 33 studies involving 12 700 participants that explored returning results to trial participants, and found that aggregate data were returned, without evaluation of what information trial participants wished to receive.17 Of the 33 studies reviewed, only 2 returned individual data to the participant, and for both of these only ‘unblinding’ was reported to the participant (not participant-level clinical and laboratory data). A single study provided both individual and aggregate results. Also, the authors noted that there was a general lack of ‘actively including patients or the public as partners in the development of the dissemination of results’. The authors noted that a weakness of their study was relying on literature reports, and this likely underestimated dissemination efforts. Shroter et al (2019) took an approach of surveying authors of published clinical trials to ascertain efforts to return clinical trial results to clinical trial participants.18 19 Questionnaires were emailed to 19 321 authors and analysed 1818 responses of authors that had enrolled individual patients. Of these, 498 (27%) had disseminated results to trial participants, but most were aggregate data (academic reports, lay reports). Of the 164 (33%) reporting that individualised data were returned, the type of individualised data was not specified. Raza et al (2019) queried the UK’s research permissions system for phase III trials for a 6-year period (2012–2017 inclusive) and found that of the 1404 phase III trials studied, 88% reported the intention to disseminate results to trial participants.20 However, only 10 of the End of Study reports cited dissemination activities, and 6 of these were through a lay summary or letter.
The primary limitation of our study was the small number of families (n=19) and their physicians (n=10) that participated in this study. The clinical trial studied was an open-label phase 2a dose-ranging and dose-finding study of 48 participants (young children with a rare genetic muscle disease; DMD), and future studies should extend our approach to larger, double-blind placebo-controlled trials in more common disorders (eg, phase 3). Future studies will also need to address potential cultural differences in attitudes of both families and their physicians based on country of origin, or other factors. Another limitation of our approach is the effectiveness of outreach (advertisement) to the parents of participating children. We had a 40% participation rate (19/48). We do not know if the 60% that did not participate was because they did not hear of the study (eg, ineffective outreach to them), or if they did not wish to participate. Our ethics approval included an ‘informational flyer’ that was meant to be distributed to clinical trial sites and provided to patient families, but sites were uncomfortable with distributing this flyer without their own institutional ethics approval. If other sponsors wish to take our centralised approach, we advise that the informational flyer for direct sponsor return of data be provided to sites for distribution to trial participants at initial contracting and ethics review and be handed to patients at initial enrolment in the clinical trial, and/or exit from the trial.
In conclusion, there is a strong desire for clinical trial participants to receive patient-level and aggregate returns of clinical trial data to them. Their treating physicians, and stakeholder foundations all uniformly acknowledge the importance of return of results to trial participants. Despite this need, it is largely unmet due to fundamental barriers (pragmatic, financial, organisational, confidentiality and ethics). We have piloted a simplified return of results process that removes most barriers, and we found that trial participants (parents of children in a trial) were highly satisfied with this novel process, and their treating physicians were also generally satisfied while expressing some reservations.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Western IRB Sponsor: ReveraGen BioPharmaSponsor Pr #: VBP15-RORIRB, Pr #: 20192458IRB, Study #: 1266745. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank the foundations that provided input, advertised this study, and enabled recruitment of participants (Muscular Dystrophy Association, Parent Project Muscular Dystrophy, World Duchenne Organisation, Foundation to Eradicate Duchenne, Little Steps Association). The authors would also like to extend thanks to the following individuals who offered advice on approach and survey questions: Edward Smith, MD, John van den Anker MD PhD, Michela Guglieri MD.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors EH is the author responsible for the overall content as the guarantor, and contributed substantially to concept and study design and drafted the manuscript. SG contributed substantially to study design, data acquisition and interpretation, and reviewed the manuscript critically. RK contributed substantially to data acquisition and interpretation and reviewed the manuscript critically. WT contributed substantially to data interpretation and presentation and reviewed the manuscript critically. HP and PC contributed substantially to concept and study design and reviewed the manuscript critically. UD contributed substantially to data interpretation and presentation and reviewed the manuscript critically. LC contributed substantially to concept and study design, data acquisition and interpretation and drafting of the manuscript.
Funding This work was supported by the National Institutes of Health (NIH) (3R44NS095423-03S1).
Competing interests UD received consultancy fees from ReveraGen Biopharma. LC is currently an employee of Johnson & Johnson, but the current work was completed while she was an employee of ReveraGen BioPharma. HP was contracted to provide expert insight into study design and interpretation of results. EH, RK and SG are employees of ReveraGen BioPharma. EH, RK and LC are stock holders in ReveraGen BioPharma. PC holds NIH, FDA and foundation grants on vamorolone clinical trials with ReveraGen BioPharma.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.