Article Text
Abstract
Introduction Ulcerative colitis (UC) is a global chronic inflammatory bowel disease, and the poor efficacy of currently available pharmacological regimens makes the management of UC a great challenge. Moxibustion has shown great potential in the management of UC. However, its effectiveness and safety are still controversial. The purpose of this study is to synthesise the latest evidence regarding the clinical efficacy and safety of moxibustion for UC.
Methods and analysis The Cochrane Library, PubMed, EMBASE, CNKI, Wanfang, VIP and SinoMed databases will be searched from inception to July 2023, to identify all randomised controlled trials with moxibustion for UC. The primary outcome will be clinical efficacy, as measured by validated scales. The serum inflammatory factor, colonoscopy results, quality of life, recurrence rate and adverse events will be the secondary outcomes. The Cochrane Risk of Bias 2.0 tool will be used to assess the methodological quality of each included trial. All data extraction will be carried out independently by two investigators. RevMan V.5.4 software will be used for data analysis and Cochran’s Q statistic and I2 test will be used to assess heterogeneity between studies. In addition, we will perform subgroup analyses, sensitivity analyses and publication bias if the available data are sufficient. The strength of evidence will be graded using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system.
Ethics and dissemination Ethics approval is not required for this review. Our findings will be published in a peer-reviewed journal.
PROSPERO registration number CRD42023425481.
- Inflammatory bowel disease
- COMPLEMENTARY MEDICINE
- IMMUNOLOGY
- Meta-Analysis
- Systematic Review
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A comprehensive literature search will be conducted in seven electronic databases covering both Chinese and English.
This protocol will be reported following the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.
The revised Cochrane Risk of Bias 2.0 Tool will be used to assess the quality of included studies.
Differences in moxibustion forms and treatment protocols may lead to a large degree of heterogeneity and make data synthesis more difficult.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) of unknown aetiology.1 It is characterised by relapsing and remitting mucosal inflammation, starting in the rectum and extending to proximal segments of the colon, with clinical manifestations mainly including abdominal pain, diarrhoea and bloody purulent stool.2 The majority of UC patients experience lifelong recurrence, which leads to significant pain and has a serious negative impact on the patient’s quality of life, mental health and job prospects.3 4 Globally, the incidence of UC is steadily increasing, with a peak age of onset between 30 and 40 years. The incidence of UC in Europe ranges from 0.6 to 24.3 per 100 000 person-years, while in North America it ranges from 8.8 to 23.1 per 100 000 person-years.5 In addition, although in the past the prevalence of UC was significantly lower in Asia than in the West, with lifestyle changes and improved diagnosis, the prevalence of UC in Asia has increased exponentially in the past decade.6–8 The rapidly increasing incidence of UC imposes significant costs on society and the healthcare system.9 The annual direct and indirect costs associated with UC are estimated to be as high as US$810–US$14.9 billion in the USA and €12.5–€29.1 billion in Europe.2 10
To date, the pathogenesis of UC remains unclear, but it is believed to be related to immune disorders, epithelial barrier defects, genetic susceptibility and environmental factors.2 5 8 Immune abnormalities are commonly thought to play a crucial role, thus therapies that modulate the immune system have become mainstream treatments. Currently, the clinical first-line therapeutic agents for UC include 5-aminosalicylic acid drugs, steroids and immunosuppressants.2 However, long-term clinical use of these drugs is limited by significant side effects such as gastrointestinal reactions, allergic reactions, nephritis and hepatitis,11 and approximately 20%–40% of patients with UC do not respond well to conventional drug therapy and require secondary drug therapy or colectomy.12 In addition, biological therapies have gained increasing attention as more specific agents in the treatment of UC in recent years. However, the durability and overall net remission rates of currently available drugs for biological therapies are relatively low,13 which limits its clinical application. As a result, concerns about inadequate response or side effects to available drugs have led many patients to seek complementary and alternative medicine (CAM) therapies.14
Among the numerous CAM therapies, moxibustion, as a widely used traditional Chinese medicine non-pharmacological therapy, has attracted great interest among UC researchers, especially in China and several parts of Asia. Moxibustion is a traditional Chinese medicine therapy that uses burning moxa sticks (the main component of moxa sticks is composed of dried leaves of Asian mugwort) to generate heat and stimulate acupoints or certain areas of the body surface, making the patient feel comfortable and warm around the moxibustion area, even the whole body.15 Moxibustion is considered a comfortable, safe, simple, low-cost and effective alternative therapy.15 16 In the past 2000 years, moxibustion has been widely used for clinical treatment in China, especially those involving pathological changes in the neuroimmunoendocrine system, such as IBD,17 18 chronic pain,19 depression20 and chronic fatigue syndrome.21
Currently, the benefits of moxibustion for UC have been widely reported22–25 and a meta-analysis of moxibustion for UC was conducted in 2010.26 However, this meta-analysis did not demonstrate the effectiveness of moxibustion due to the limited number and low quality of included studies. In addition, two meta-analysis protocols involving moxibustion for UC have been published recently.27 28 However, these studies focused on specific types of moxibustion (herb-partitioned moxibustion or heat-sensitive moxibustion), which limits the generalisability of the findings of both studies. In order to ensure the universality of the research evidence as well as maximise the number of potentially eligible studies included, moxibustion in this study will include all types of traditional moxibustion therapies. In addition, a growing number of randomised controlled trials (RCTs) have investigated the efficacy of moxibustion for UC in recent years,29 30 and integrating these eligible trials into the latest meta-analyses to update the previous research evidence is necessary. Therefore, we conducted this study to evaluate the available evidence from RCTs to investigate the effectiveness and safety of moxibustion for UC.
Methods
Study registration
This review has been registered at PROSPERO (No. CRD42023425481). This protocol is reported following the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.31
Eligible criteria
Study designs
Only RCTs evaluating the effectiveness and safety of moxibustion for UC will be included in this review. There are no restrictions on language or time of publication. Animal experiments, non-RCTs and replicated studies will not be considered.
Participants
We will include adult subjects with a confirmed diagnosis of UC based on clear diagnostic criteria, such as those from Europe32 or China.33 There are no restrictions on gender, disease duration or severity. Also, patients with other intestinal diseases with symptoms similar to UC, such as acute gastroenteritis and irritable bowel syndrome, will be excluded.
Interventions
The only permitted experimental treatment is moxibustion, which can be used either alone or in combination with the same western drugs as in the control group. In order to minimise the heterogeneity among the included studies from a clinical perspective, moxibustion here will be restricted to traditional moxibustion therapies in which the treatment is performed by burning moxa materials, such as thermal moxibustion, mild moxibustion, thunder-fire moxibustion, indirect moxibustion, etc. Differences in the duration and frequency of moxibustion treatments as well as the material of the moxa sticks will not be taken into account.
Comparators
Control measures will be conventional Western medications such as glucocorticoids, immunomodulators and biologics, with detailed reporting of the dosage, method and duration of treatment. Studies comparing moxibustion at different acupoints, different moxibustion methods or comparing moxibustion with other non-pharmacological therapies will be excluded.
Types of outcomes
Primary outcome
The primary outcome will be clinical efficacy as assessed by validated scales, including the Mayo score or Disease Activity Index.
Secondary outcomes
Serum inflammatory factor levels (eg, TNF-α, IL-6, IL-8).
Colonoscopy results.
Quality of life measured using a validated instrument such as the Inflammatory Bowel Disease Questionnaire.
Recurrence rate.
Adverse events.
Search strategy
We will perform a systematic literature search in three English electronic databases (PubMed, Cochrane Library and EMBASE) and four Chinese electronic databases (Wanfang database, Chinese National Knowledge Infrastructure, VIP and SinoMed). All online databases will be searched for eligible RCTs from database establishment to July 2023. The database search will be conducted using a combination of medical search headings and free words, with the search terms including disease name (eg, UC), intervention (eg, moxibustion) and study design (randomised clinical trial). All possible combinations of search terms will be used to ensure the comprehensiveness of the included studies. In addition, references of all included studies will be manually screened to identify potentially eligible trials. The search strategy is described in online supplemental file 1.
Supplemental material
Study selection
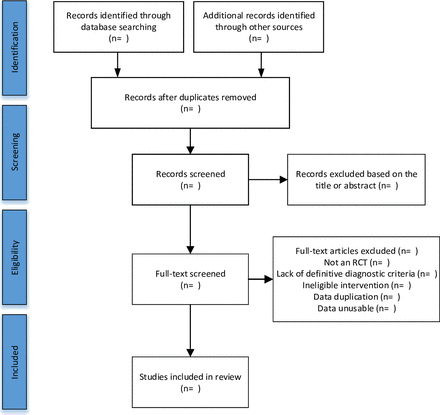
Articles retrieved from the seven databases will be imported into EndNote V.X9 software to remove duplicate records. Two independent investigators (YL and WZ) will screen eligible studies by reviewing article titles, abstracts and full text. Any disagreements during the study selection process will be resolved in consultation with a third investigators (J.Y). The flow chart of the study selection is shown in figure 1.
Flow diagram of the study selection process. RCT, randomised controlled trial.
Data extraction and management
Two independent reviewers (YL and WZ) will extract the following information from the eligible studies: basic details of study (first author, country, publication year, language, journal and article title), basic information of participants (sample size, diagnostic criteria, average age, gender distribution and duration of UC), intervention details (type, frequency and duration of moxibustion), details of the comparator (drug name, dose, frequency and duration of treatment), methodological characteristics (study design, randomisation, allocation and blinding) and outcomes (primary and secondary outcomes). A self-designed form (online supplemental file 2) based on the Standards for Reporting Interventions in Clinical Trials of Moxibustion checklist will be used for data collection.34 The corresponding author will be contacted for additional information if data are insufficient or ambiguous. A third independent researcher (JY) will resolve any disagreements raised.
Supplemental material
Assessment for risk of bias
Two independent investigators (YL and WZ) will determine the methodological quality of the included RCTs according to Cochrane’s Risk of Bias 2.0 (ROB 2.0) tool.35 The ROB for included studies will be rated according to the following five domains: (1) process of randomisation, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome and (5) selection of the reported results. The ROB for each domain will be judged as ‘high risk’, ‘some concerns’ or ‘low risk’ and the overall ROB for the study will be given accordingly. Any disagreement between the investigators will be resolved through consultation with a third independent investigator (JY).
Data analysis
Data synthesis
Data analysis will be performed using RevMan (V.5.4) software. Risk ratios with 95% CIs will be used to calculate dichotomous data, and weighted mean difference (WMD) or standardised mean difference (SMD) with a 95% CI will be used to analyse continuous data. Results measured on the same assessment instrument or same scale will use WMD; otherwise, SMD will be used. Dichotomous results will be analysed using the Mantel-Haenszel method, while continuous data will be pooled using the inverse variance method.36 37
Assessment of heterogeneity
Statistical heterogeneity among the included trials will be assessed using Cochrane’s Q-test and the Inconsistency Index (I2).38 Heterogeneity between studies will be considered acceptable if I² <50% and p>0.1, and a fixed-effects model will be used for pooled analyses. Conversely, significant heterogeneity between trials is indicated, and subgroup analyses or sensitivity analyses will be performed to explore potential sources of heterogeneity. If clinical and methodological homogeneity is maintained but statistical heterogeneity persists, a more conservative assessment of intervention effects will be conducted using a random effects model. Narrative analyses will be conducted when a combined data analysis is not feasible.
Subgroup analysis and sensitivity analysis
If significant heterogeneity exists among the included studies and sufficient data are available, subgroup analyses will be performed for different moxibustion interventions (eg, moxibustion and moxibustion combined with drugs), moxibustion type (eg, mild moxibustion, thunder-fire moxibustion, indirect moxibustion), treatment duration and disease severity. If subgroup analyses failed to explain the source of heterogeneity, sensitivity analyses will be performed to assess the robustness of the combined results by excluding studies with high ROB or using different statistical models.
Assessment of publication bias
If more than 10 RCTs are available for meta-analysis, funnel plots will be generated to assess publication bias. If asymmetry is observed, the Egger’s regression test will be used for quantitative assessment.39
Grading the quality of evidence
The GRADE system will be applied to assess the certainty of evidence.40 We will decide whether to downgrade the quality of evidence based on five domains of study limitations (risk of bias, inconsistency, indirectness, imprecision and publication bias). According to the GRADE rating criteria, the quality of evidence will ultimately be rated as ‘very low’, ‘low’, ‘moderate’ or ‘high’.
Patient and public involvement
No patient participation.
Ethics and dissemination
No ethical approval is required for this review. Our findings will be submitted to peer-reviewed journals.
Discussion
UC has been recognised as a global disease and is particularly prevalent in Western countries.4 41 Due to the inflammatory nature of UC, if not effectively treated, it may exacerbate bowel damage and increase the risk of surgery and colorectal cancer.25 In the field of CAM, patients with IBD have one of the highest rates of CAM use, with nearly 21%–60% of patients with IBD reported to have used CAM.14
In China, moxibustion is the most widely used form of non-pharmacological treatment. Previous studies have revealed that moxibustion can effectively control bowel inflammation by regulating the physiological balance at multiple links and targets in the body and has the advantages of safety, good long-term efficacy and low recurrence rate.42 Therefore, moxibustion has been widely used in the clinical management of patients with UC. However, the benefits of moxibustion for UC have not been supported by high-level evidence, and its potential value for clinical application has not been fully revealed.26 Therefore, a meta-analysis of existing RCTs is necessary.
Our current study will summarise the latest evidence on the effects of moxibustion on UC. The findings of this study will hopefully provide an evidence base for clinicians, policy-makers and healthcare administrators to develop a moxibustion treatment protocol in clinical practice. To a certain degree, this study will help to fulfil the current evidence gap of moxibustion for UC and contribute to evidence-based treatment decisions. Additionally, this study will also provide a reference for further study design and guide new trials to fill the current evidence gaps. Inevitably, this study may have some limitations. First, differences in moxibustion methods, acupoint selection, treatment frequency and duration in the included studies may lead to a high heterogeneity. Second, the methodological quality of the included RCTs may affect the accuracy of the findings in this study.
Supplemental material
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Conception or design of the study: YL and JY. Data collection, data analysis and interpretation: WZ, WC and BD. Writing the manuscript: YL and JY. Critical revision of the paper: YL,WZ and JY. Approval of the final draft: YL, WZ, WC, BD and JY.
Funding This study is supported by the Science and Technology Research Project of Jiangxi Provincial Department of Education (grant number GJJ218916 and GJJ218919) and Natural Science Foundation of Jiangxi Province (grant number 20224BAB216110).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.