Article Text
Abstract
Objective This review aims to provide an estimate of sarcopenia prevalence and its impact on clinical characteristics in patients with systemic sclerosis (SSc).
Design Systematic review and meta-analysis.
Data sources Embase, Medline, Web of Science and the Cochrane Central Register of Controlled Trials were systemically searched from inception to 24 May 2023.
Eligibility criteria for selecting studies We included observational studies that reported the prevalence of sarcopenia in patients with SSc.
Data extraction and synthesis Two reviewers independently performed study selection and data extraction using standardised methods. Risk of bias was assessed using the Agency for Healthcare Research and Quality Scale and the Newcastle–Ottawa Scale. Meta-analysis was conducted using random effects models.
Results A total of 4583 articles were screened and 9 studies with data from 815 patients were included in the analysis (8 cross-sectional studies and 1 retrospective cohort study). The overall prevalence of sarcopenia in patients with SSc was 22% (95% CI 17% to 28%). Patients with SSc with sarcopenia had a poorer quality of life (mean difference −12.02; 95% CI −19.11 to −4.93) and higher C reactive protein (CRP) levels (standardised mean difference 0.67; 95% CI 0.35 to 1.00).
Conclusions Sarcopenia is common in patients with SSc. Patients with SSc with sarcopenia had a worse quality of life and higher CRP levels, based on our findings. Given the detrimental impact of sarcopenia on quality of life, future efforts aimed at early identification of sarcopenia in the clinical assessment of patients with SSc may have significance.
PROSPERO registration number CRD42022368326.
- Rheumatology
- GERIATRIC MEDICINE
- Systematic Review
Data availability statement
Data are available upon reasonable request. The data are accessible upon reasonable request from the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the first systematic review and meta-analysis to evaluate the prevalence and impact of sarcopenia in patients with systemic sclerosis (SSc).
We conducted a comprehensive literature search to ensure that all eligible studies were included in the analysis.
We could not establish a definitive causal relationship between sarcopenia and SSc.
Even though this review included studies from different continents (Europe, South America and Asia), data on participant race were not accessible, limiting its potential applicability to specific patient subgroups.
Introduction
Systemic sclerosis (SSc) is a rare immune-mediated rheumatic disease that is characterised by inflammation, microvascular damage and progressive fibrosis of both the skin and internal organs, such as the gastrointestinal tract, lung, heart and kidney.1 2 Depending on the extent of cutaneous involvement, SSc can be classified as limited cutaneous SSc (lcSSc) or diffuse cutaneous SSc (dcSSc).3 Patients with SSc are at risk for body composition abnormalities, including loss of skeletal muscle mass, due to malnutrition resulting from gastrointestinal involvement, chronic inflammation and steroid therapy.4–7 In addition, heart, lung and joint involvement in patients with SSc can lead to impaired exercise ability and decreased physical activity.8 These factors are closely related to sarcopenia, which is an age-related disease characterised by progressive and generalised loss of skeletal muscle mass and strength.9 The coexistence of sarcopenia and SSc can exacerbate the patient’s health issues and increase their healthcare costs, posing significant challenges for healthcare professionals.
According to a meta-analysis, the prevalence of sarcopenia in community-dwelling elders aged over 60 years was 11% (95% CI 8% to 13%) in men and 9% (95% CI 7% to 11%) in women.10 The presence of sarcopenia increases the risk of falling, functional decline, frailty and mortality, leading to poor quality of life and significant healthcare expenses.11 The high prevalence of sarcopenia in older adults, combined with its detrimental consequences, warrants the need for effective prevention and management strategies. In patients with SSc, addressing sarcopenia may improve their functional status and overall health outcomes, highlighting the importance of early screening and intervention. Healthcare professionals need to recognise the interplay between SSc and sarcopenia to provide optimal care for these patients.
In recent years, the presence of sarcopenia in SSc has garnered attention in several studies.4–7 12–16 The documented prevalence of sarcopenia in SSc varies widely from 10.7% to 42% among different studies, which can be attributed to several factors.4 5 Differences in diagnostic criteria and assessment methods used in various studies, such as those proposed by the European Working Group of Sarcopenia in Older People (EWGSOP)9 17 and the Asian Working Group for Sarcopenia (AWGS),18 can result in variations in the evaluation of muscle mass in patients. Furthermore, the influence of sarcopenia on the clinical features of patients with SSc has been a topic of debate. For instance, Caimmi et al12 suggested that individuals with SSc and sarcopenia had a longer duration of disease; the longer disease duration means that patients live longer with the disease, while Siegert et al6 contradicted this claim and found no difference between sarcopenia and disease duration in patients with SSc.
Currently, no comprehensive systematic review or meta-analysis has examined sarcopenia in SSc. Therefore, we conducted a systematic review and meta-analysis to identify the diagnostic criteria for sarcopenia and evaluate the most reliable evidence on the prevalence of sarcopenia in patients with SSc, as well as the effect of sarcopenia on the clinical features of patients with SSc.
Methods
Data sources and search strategy
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline19 and registered in PROSPERO (CRD42022368326). We systemically searched four electronic databases, including Embase, Medline, Web of Science and the Cochrane Central Register of Controlled Trials, to identify all relevant articles relating to sarcopenia and SSc, without language restrictions. Our search encompassed all records published from inception to 24 May 2023, using the following terms: ‘systemic sclerosis’, ‘scleroderm*’, ‘SSc’, ‘muscular atrophy’, ‘sarcopen*’ and ‘myopen*’ (online supplemental tables S1–S4). Additionally, we conducted a manual search of the reference lists of the included articles to identify potential studies that may have been overlooked by the principal search.
Supplemental material
Inclusion and exclusion criteria
The following inclusion and exclusion criteria were employed for this systematic review and meta-analysis: (1) studies conducted exclusively on adult patients (age >18 years) diagnosed with SSc; (2) studies reporting the prevalence of sarcopenia in patients with SSc; (3) studies defining sarcopenia as low muscle mass (LMM) plus low muscle strength (LMS) and/or low physical performance (LPP) or LMM alone; LMM was evaluated by dividing appendicular skeletal muscle mass (in kilograms) by height in metres squared, LMS by hand grip strength, LPP by gait speed (GS) or Short Physical Performance Battery (SPPB) and diagnostic cut-offs varied depending on the criterion9 17 18 20; (4) studies measuring lean mass or muscle mass using one of the four main techniques: dual-energy X-ray absorptiometry, bioelectrical impedance analysis, MRI and CT and (5) observational studies. Conversely, the exclusion criteria were as follows: repeated studies (defined as either identical data or identical articles).
Outcomes
The main outcomes of this systematic review comprise two aspects: first, the prevalence of sarcopenia among patients with SSc, and second, the clinical features of patients with SSc who suffer from sarcopenia compared with those who do not. These clinical features encompassed a range of factors, namely, the duration of disease, the quality of life assessed by the Short Form-36 (SF-36) survey,21 the pulmonary function (the forced vital capacity (FVC)-predicted value) and the C reactive protein (CRP) level. These features are frequently the focus of clinical studies in patients with SSc, and it is of significant interest to understand how sarcopenia impacts them.
Study selection and data extraction
After removing duplicates, the studies identified through the search strategy underwent eligibility assessment by two reviewers (XT and TL), who independently screened the titles and abstracts and assigned them to one of three categories: ‘include’, ‘exclude’ or ‘maybe’. Subsequently, the full-text articles of those categorised as ‘include’ or ‘maybe’ were reviewed to arrive at a final selection, with any discrepancies between the reviewers resolved by a third reviewer (JY). Two reviewers (XT and XS) independently extracted the following variables using a predefined data collection form: first author, publication year, country, study design, sample size, mean age, number of females, disease subtype, mean disease duration, SSc diagnostic criteria, sarcopenia diagnostic criteria, assessment method for detecting sarcopenia and prevalence of sarcopenia. Additionally, we also collected data on clinical features in the form of mean±standard deviation (SD). For those studies that were not expressed as mean±SD, we performed data conversion with the method recommended by Luo et al22 and Wan et al.23
Assessment of quality
Two authors (XT and TJ) independently assessed the quality of the included studies using the Agency for Healthcare Research and Quality (AHRQ)24 Scale in cross-sectional studies. This tool consists of 11 questions, with a ‘no’ or ‘unclear’ receiving 0 points and a ‘yes’ receiving 1 point. Low-quality articles received scores of 0–3, moderate-quality scores of 4–7 and high-quality scores of 8–11. The Newcastle–Ottawa Scale (NOS) was used to judge the quality of the cohort study.25 The NOS scoring system assigns points from 0 to 9. We assigned values ranging from 0 to 3, 4 to 6 and 7 to 9 for low, moderate and high-quality, accordingly. Any discrepancies were resolved through discussion or consensus with a third author (JY.).
Statistical analysis
The prevalence of sarcopenia in patients with SSc was determined by calculating the proportion of patients with sarcopenia in each study and conducting a meta-analysis of single proportions. We performed this meta-analysis using Stata/SE (V.12.0, StataCorp, College Station, Texas, USA). Forest plots were used to illustrate the prevalence of sarcopenia, along with the corresponding 95% confidence intervals (CIs) for each study and the overall estimate. Clinical characteristics such as disease duration, the SF-36 value, the FVC-predicted value and the CRP level from studies that compared patients with SSc with and without sarcopenia were also analysed using Review Manager (V.5.4, The Cochrane Collaboration, Oxford, UK) and expressed as mean difference (MD) or standardised mean difference (SMD) with 95% CI. Heterogeneity across studies was assessed via the I2 statistic, with values of 25% being considered low, 50% moderate and 75% high.26 Considering the variation in the definition of sarcopenia, diagnostic criteria and population characteristics among the included studies, this study employed a random-effects model.
Subgroup analyses were conducted to investigate potential sources of heterogeneity, focusing on sarcopenia definition (1 vs >1 diagnostic criteria), disease subtype and mean age (<60 vs ≥60 years). The reasons for grouping in subgroup analysis are as follows. First, variability in the definition of sarcopenia will result in varied prevalence estimates for patients with SSc. Unsurprisingly, increasing the number of necessary criteria in a sarcopenia definition will eventually diminish sarcopenia prevalence. Additionally, the disease subtype is an important factor that affects the prevalence of sarcopenia. Patients with dcSSc are more prone to develop sarcopenia.14 Moreover, age is an essential factor that influences the onset and course of sarcopenia, with the prevalence of sarcopenia increasing with age. Meta-regressions were also conducted on sample size, mean age, percentage of female patients and duration of SSc. However, due to limited data on the clinical characteristics of patients with SSc with and without sarcopenia, subgroup analyses and meta-regressions were not conducted. To evaluate the stability of pooled results, sensitivity analysis was performed by excluding one study at a time. Publication bias was evaluated using Egger’s test.27 Statistical significance was set at p<0.05 for all analyses.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
Search results
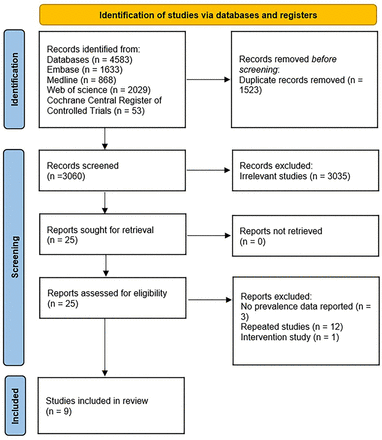
A comprehensive search of databases yielded 4583 articles. After eliminating duplicates (n=1523), the remaining 3060 titles and abstracts were screened. Subsequently, 25 relevant articles underwent full-text reading, and 16 were excluded for reasons specified in the flow chart and online supplemental table S5. Ultimately, nine studies were eligible for inclusion in this meta-analysis (figure 1).
The flow chart of the literature selection.
Study characteristics
Online supplemental table S6 provides an overview of the characteristics of the studies included in this meta-analysis. A total of 815 patients with SSc from nine eligible studies4–7 12–16 published between 2018 and 2022 were included. The mean age of the patients ranged from 52.5 to 64.1 years, while the mean duration of SSc ranged from 6 to 12.8 years. The majority of the studies (eight out of nine) had a cross-sectional design,4–6 12–16 with one being a retrospective cohort study.7 The studies were conducted in various regions, with five from Europe,5–7 12 16 two from South America13 15 and two from Asia.4 14
Risk of bias
According to the AHRQ and NOS ratings, eight of the eligible studies4–7 12 14–16 were of moderate quality, with only one article13 classified as high quality (online supplemental tables S7 and S8).
Methods used to assess sarcopenia
Online supplemental table S6 provides an overview of the diagnostic criteria used to evaluate sarcopenia across the included studies. Among them, seven studies4–7 13 15 16 employed EWGSOP criteria (five EWGSOP 2010 and two EWGSOP 2019) while one14 used AWGS criteria. Three studies5 7 12 solely relied on LMM for sarcopenia diagnosis, while six studies4 6 13–16 used LMM combined with LMS and/or LPP. The sarcopenia diagnostic criteria and cut-off values in the studies are summarised in table 1. Muscle mass was measured using dual-energy X-ray absorptiometry in seven studies5 7 12–16 and bioelectrical impedance analysis in two studies.4 6 Handgrip dynamometry was used to assess muscle strength in six studies,4 6 13–16 while GS (three studies14–16) and the SPPB (two studies13 16) were used to evaluate physical performance.
Criteria and cut-off points used to detect sarcopenia in each study
Sarcopenia prevalence
Overall sarcopenia prevalence
The nine studies included in this review reported the prevalence of sarcopenia in patients with SSc, ranging from 10.7% to 42% (online supplemental table S6). The pooled prevalence of sarcopenia in patients with SSc was estimated at 22% (95% CI 17% to 28%), as shown in figure 2.
The pooled prevalence of sarcopenia in patients with systemic sclerosis.
Subgroup analysis of sarcopenia prevalence
The prevalence of sarcopenia differed in studies that used a single criterion (LMM; 28% (95% CI 16% to 42%)) versus those that employed >1 criterion (LMM+LMS and/or LPP; 20% (95% CI 15% to 25%)), with no statistically significant difference noted (p=0.234, online supplemental figure S1). Subgroup analysis based on disease subtype revealed that sarcopenia prevalence in dcSSc (30% (95% CI 23% to 37%)) was higher than that in lcSSc (23% (95% CI 12% to 36%)), and the difference was not statistically significant (p=0.339, online supplemental figure S2). The United Nations defines an older person as someone above the age of 60. Therefore, we also performed a subgroup analysis stratified by the mean age of the participants, with <60 and ≥60 years as the cut-off points. The prevalence of sarcopenia was lower in patients younger than 60 years (20% (95% CI 12% to 29%)) versus those older than 60 years (24% (95% CI 17% to 32%)), but the difference was not of statistical significance (p=0.539, online supplemental figure S3).
Meta-regression analyses
The results of the meta-regression analyses indicated that there was no significant association between the prevalence of sarcopenia and sample size (p=0.424), mean age of patients (p=0.532), the proportion of female patients (p=0.449) or duration of SSc (p=0.255). These findings are summarised in online supplemental table S9.
Impact of sarcopenia on the clinical characteristics of patients with SSc
Duration of SSc
Data from a total of four studies comprising 511 patients were included in the meta-analysis of SSc duration, which revealed that individuals with sarcopenia did not have a longer disease duration than those without sarcopenia (MD 2.97 years (95% CI −0.13 to 6.08); I2=90%, figure 3A).
Impact of sarcopenia on clinical characteristics in patients with SSc. CRP, C reactive protein; FVC, forced vital capacity; SF-36, Short Form-36; SSc, systemic sclerosis.
Quality of life
The meta-analysis included two studies with a total of 191 patients, which provided data on the SF-36 value. The findings of the meta-analysis indicated that patients with sarcopenia had a lower SF-36 value compared with those without sarcopenia (MD −12.02 (95% CI −19.11 to −4.93); I2=71%, figure 3B), that is, having sarcopenia was associated with poorer quality of life compared with those without sarcopenia.
Pulmonary function
The meta-analysis incorporated two studies involving a total of 320 patients that reported data on the FVC-predicted value. The results indicated that patients with sarcopenia did not have a lower FVC-predicted value than those without sarcopenia (MD −4.02% (95% CI −8.67 to 0.62); I2=0%, figure 3C). Therefore, there was no significant difference in pulmonary function between patients with sarcopenia and non-sarcopenia.
CRP level
Data from two studies comprising 191 patients were analysed to investigate the relationship between sarcopenia and CRP level. The results showed that sarcopenia was associated with a higher CRP level than no sarcopenia (SMD 0.67 (95% CI 0.35 to 1.00); I2=0%, figure 3D).
Sensitivity and publication bias analysis
The sensitivity analysis revealed that the overall prevalence of sarcopenia was not significantly affected by any individual study (online supplemental figure S4). In addition, Egger’s test suggested no publication bias in this review (p=0.311, online supplemental figure S5).
Discussion
Primary results
In this meta-analysis encompassing nine studies, the pooled prevalence of sarcopenia among 815 patients diagnosed with SSc was estimated to be 22%, which was significantly greater than that in community-dwelling older adults.28 Notably, patients with SSc diagnosed with sarcopenia had poorer quality of life and a higher CRP level, while no significant difference was noted for disease duration and FVC-predicted value when compared with patients without sarcopenia.
Mechanism basis
Sarcopenia, a condition characterised by loss of muscle mass and function, can be age-associated (primary sarcopenia) or secondary to chronic diseases, including malignant tumours and musculoskeletal diseases.29–31 Compared with other chronic inflammatory rheumatic diseases, sarcopenia has not been extensively evaluated in SSc. Recently, some studies have focused on the presence of sarcopenia in SSc. Nevertheless, the pathogenesis of sarcopenia in SSc remains unclear. Possible mechanisms contributing to the development of sarcopenia in SSc include (1) malnutrition: gastrointestinal involvement is the most frequent internal complication of SSc.32 Symptoms such as oesophageal reflux, early satiety, nausea and vomiting may lead to reduced caloric intake.12 Additionally, fibrosis of the bowel wall and small intestine bacterial overgrowth can result in malabsorption of nutrients. Therefore, malnutrition is prevalent in patients with SSc. One study in community-dwelling older adults demonstrated that malnutrition is an independent predictor of sarcopenia (OR 2.42; 95% CI 1.04 to 5.60).33 (2) Oxidative stress and chronic inflammation: oxidative stress, which is an imbalance in oxidant and antioxidant levels, is commonly observed in patients with SSc.34 Increased oxidative stress disrupts the balance between the degradation and resynthesis of skeletal muscle proteins.35 In addition, chronic low-grade inflammation is detrimental to skeletal muscle in humans.36 Inflammatory cytokines, such as tumour necrosis factor-α and interleukin-6, have been reported to contribute to the pathogenesis of SSc.37 These cytokines stimulate protein catabolism and suppress muscle synthesis, ultimately leading to muscle wasting.38 (3) Physical inactivity: due to pain and joint involvement, physical inactivity is common in patients with SSc,39 leading to faster and greater muscle loss.11 However, the mechanism of sarcopenia in patients with SSc remains to be confirmed by future research.
Interpretation of the results
This review offers unique insight into sarcopenia in patients with SSc. It describes the prevalence of sarcopenia in patients with SSc and how it is impacted by the different definitions of sarcopenia. The varying prevalence of sarcopenia may be explained in part by the variety of definitions. However, there was no statistical difference between 1 and >1 diagnostic criteria. This might be due to the lack of robustness of the combined results as a result of the small number of studies using one diagnostic criterion. In addition, discrepancies in sarcopenia diagnostic cut-offs among the included studies may have resulted in differing sarcopenia prevalence. Furthermore, our meta-analysis indicated no statistically significant variation in the prevalence of sarcopenia between disease subtypes, which is consistent with the results of Sangaroon et al.14 It is important to note that this conclusion needs to be interpreted with caution due to the limited number of studies that could be included in the analysis. Although sarcopenia commonly occurs as an age-related process in older individuals,11 it becomes more common as people get older. Our meta-analysis demonstrated that the difference in the prevalence of sarcopenia was not statistically significant between the patients over 60 years old and the patients under 60 years old. Furthermore, patients younger than 60 years old all used >1 criterion to diagnose sarcopenia, which makes the prevalence of sarcopenia in young people even lower. This suggests that, despite the influence of age on the presence of sarcopenia, the illness itself is responsible for sarcopenia onset and progression in patients with SSc. Therefore, rheumatologists should screen for sarcopenia even in young patients with SSc. However, this conclusion must be confirmed by a large number of high-quality clinical studies.
Our meta-analysis also revealed that patients with SSc diagnosed with sarcopenia had a poorer quality of life. On the one hand, involvement of the heart, lungs and joints in patients with SSc might result in diminished exercise capacity and decreased physical activity,8 making patients with SSc vulnerable to sarcopenia. On the other hand, sarcopenia is associated with a variety of negative outcomes, including hospitalisation, functional decline, falls and death.40 41 Therefore, it should come as no surprise that patients with SSc with sarcopenia have a higher risk of having a worse quality of life. Furthermore, individuals with SSc who had sarcopenia had higher CRP levels, according to our findings. This result is not surprising given that chronic inflammation is a known contributor to secondary sarcopenia.42 However, our review indicated that no significant difference was noted for disease duration or FVC-predicted value between patients with SSc with and without sarcopenia. According to the results of Caimmi et al,12 the longer the disease duration, the greater the risk of sarcopenia. This might be due to the minimal number of studies that could extract data, resulting in false negatives in the pooled study results. Therefore, large prospective cohort studies are required to confirm this conclusion.
Clinical implications
This meta-analysis provides a comprehensive evaluation of the prevalence, diagnostic criteria and impact of sarcopenia in patients with SSc, which has not been previously done. The results of this study provide an up-to-date estimation of the prevalence of sarcopenia, which can guide sample size calculations for future research. While sarcopenia has been relatively understudied in SSc compared with other rheumatic diseases, our findings suggested that neither sarcopenia definition, disease subtype nor age affects the prevalence of sarcopenia. Patients with SSc with sarcopenia had a poorer quality of life, according to our findings. Therefore, early identification and intervention of patients with sarcopenia by clinicians is crucial. The high prevalence of sarcopenia in patients with SSc highlights the importance of early screening and management. Standardised criteria for sarcopenia diagnosis are also essential in patients with SSc to minimise variations in prevalence. These findings have important implications for future research, clinical practice and policy development in managing sarcopenia in patients with SSc and can potentially improve outcomes for these patients.
Strengths and weaknesses
This systematic review undertook a comprehensive and meticulous literature search to ensure that all pertinent studies were included in the analysis. The selection of studies, data extraction and quality assessments were carried out independently by two reviewers, thereby enhancing the accuracy and reliability of the results. Subgroup analyses and meta-regression analyses were also conducted to explore the possible sources of heterogeneity, while sensitivity and publication bias analyses were performed to ensure robust and dependable conclusions.
Nevertheless, we must acknowledge certain limitations of our study. First, since most of the included studies were cross-sectional, it is impossible to establish a definitive causal relationship between sarcopenia and SSc. Nonetheless, this is a limitation inherent to the original literature and beyond our control. We, therefore, look forward to high-quality prospective cohort studies to provide more conclusive evidence on this matter. Second, there was some heterogeneity among the included studies in terms of factors such as the definition of sarcopenia, measurement approaches and diagnostic cut-offs. Moreover, most of the studies had small sample sizes. Therefore, future studies should aim to use uniform diagnostic criteria for sarcopenia and expand the sample size to improve the quality of research. Finally, even though this review included studies from different continents (Europe, South America and Asia), data on participant race were not accessible, limiting its potential applicability to specific patient subgroups.
Conclusions
Sarcopenia is common in patients with SSc. Patients with SSc with sarcopenia had a worse quality of life and higher CRP levels, based on our findings. Given the detrimental impact of sarcopenia on quality of life, future efforts aimed at early identification of sarcopenia in the clinical assessment of patients with SSc may have significance.
Data availability statement
Data are available upon reasonable request. The data are accessible upon reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors All authors conceived and designed this review; YJ, XT and JY developed the search strategy; XT and TL screened studies; XT and XS extracted data; XT and TJ appraised study quality; XT and NG conducted data analysis; XT drafted the manuscript; all authors revised the manuscript for important intellectual content. JY had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. JY is responsible for the overall content as the guarantor. JY accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding This study was supported by grants from the National Key Research and Development Program (2020YFC2009004), Sichuan Science and Technology Program (2022YFS0295, 2022YFG0205, 2023ZYD0173), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21005) and Health Research of Cadres in Sichuan province (SCR2022-101).
Disclaimer The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.