Article Text
Abstract
Objectives Patients with inflammatory bowel disease (IBD) may experience comorbidities involving metabolic syndrome (MetS). However, this association remains controversial. Our objective was to estimate the prevalence of MetS in patients with IBD and assess whether MetS is more strongly associated with ulcerative colitis (UC) or Crohn’s disease (CD).
Design Systematic review and meta-analysis.
Data sources PubMed, Cochrane Library, Web of Science, EMBASE and MEDLINE were searched from their inception to July 2022.
Eligibility criteria Observational studies reporting data regarding the rate of comorbid MetS among patients with IBD and published in English.
Data extraction and synthesis The Preferred Reporting Items for Systematic Reviews and Meta-Analyses and the Meta-analysis of Observational Studies in Epidemiology reporting guidelines were followed. Pooled prevalence, ORs and 95% CIs were calculated using random-effects models. The Newcastle-Ottawa Scale and the Agency for Healthcare Research and Quality checklist were used. Heterogeneity, sensitivity and stratified analyses were performed using R (V.4.2.1).
Results 11 eligible studies involving 2501 patients were included. Of these studies, four reported MetS prevalence separately by IBD phenotype, and only one contained a non-IBD comparison group. Overall, the methodological quality of the included studies was moderate. The pooled prevalence of MetS in IBD was 19.4% (95% CI 15.1% to 23.8%), with a moderate heterogeneity (I2=51.8%, Cochrane Q statistic=12.4, p=0.053). Stratified analyses demonstrated that the aggregate estimate of comorbid MetS was significantly higher in UC than in CD (38.2% vs 13.6%, χ2=4.88, p=0.03). We found a positive association between MetS and UC compared with CD (OR=2.11, 95% CI 1.19 to 3.74, p=0.01). Additionally, four studies identified that higher age was a risk factor associated with the development of MetS.
Conclusions MetS is not rare in IBD, especially in UC. However, longitudinal studies are needed to further clarify the relationship between IBD and MetS.
PROSPERO registration number CRD42022346340.
- Inflammatory bowel disease
- QUALITATIVE RESEARCH
- Systematic Review
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Our study was registered on PROSPERO and represents a comprehensive synthesis of the available evidence on the prevalence and association of comorbid metabolic syndrome (MetS) among patients with inflammatory bowel disease (IBD).
The Newcastle-Ottawa Scale and the Agency for Healthcare Research and Quality checklist were used to assess the quality of individual studies, while the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and the Meta-analysis of Observational Studies in Epidemiology guidelines were followed in reporting the results.
Heterogeneity, sensitivity and stratified analyses were performed.
Most of the studies included in this meta-analysis were cross-sectional in design, and some potential confounding factors could lead to bias in the association between MetS and IBD.
The number of studies included in the subgroup analyses was limited.
Introduction
Metabolic syndrome (MetS) is a pathological condition characterised by abdominal obesity, insulin resistance, hypertension and hyperlipidaemia.1 The changing lifestyle of modern people, lack of exercise and excessive accumulation of calories are suggested to be the direct causes of this kind of disease.2 While with deeper cognition about MetS, it is found to be associated with many chronic diseases, like type 2 diabetes, coronary diseases, stroke and other disabilities. MetS has increased the social burdens including the cost of healthcare and potential loss of economic.1 3
Inflammatory bowel disease (IBD) causes idiopathic chronic inflammation of the intestines, with the aetiology unknown and with the incidence increasing worldwide. In the 21st century, the incidence of IBD is more than 0.3% of the total population in Western countries such as in the UK, the USA, Canada, Denmark, Sweden, Germany and Australia, and is also rising in developing countries.4 Crohn’s disease (CD) and ulcerative colitis (UC) are two major phenotypes of IBD. The aetiology of IBD (UC or CD) is yet to be elucidated. Currently, IBD is considered a multifactorial disease involving genetic predisposition, environmental factors and immunometabolic disorders.5 6
Interestingly, there are many commonalities between IBD and MetS, such as dyslipidaemia, immune system imbalance and chronic inflammation state.3 Many previous studies have shown an overlap between IBD and MetS and have investigated the prevalence rates. However, the results are diverse.6–8 Prior studies looking at the relationship between IBD and MetS have been observational studies or from a single centre limited by sample size. The aim of this systematic review and meta-analysis was to determine the overall prevalence of comorbid MetS among patients with IBD and to explore the association.
Methods
This meta-analysis is reported in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology.9 10 The protocol was registered with PROSPERO (CRD42022346340).
Search strategy
We searched PubMed, Cochrane Library, Web of Science, EMBASE and MEDLINE from the respective dates of database inception to July 2022 for studies reporting the prevalence of comorbid MetS among patients with IBD. A combination of medical subject heading terms and/or free text words was used: “metabolic syndrome”, “Inflammatory bowel disease”, “Ulcerative colitis”, “Crohn disease”, “MetS”, “MS”, “IBD”, “UC” and “CD”. In addition, we also conducted hand-searching of all references of the retrieved studies for further relevant reports. The search was limited to papers published in English. No other restrictions were imposed. The search strategy was undertaken independently by two investigators (YL and MZ) who are experienced in information retrieval. The preliminary search strategy is shown in online supplemental table 1 and was adapted according to syntax-related requirements of the electronic databases.
Supplemental material
Study selection
The inclusion criteria of eligible studies were as follows: (1) patients with confirmed IBD (including UC and CD) and MetS; (2) observational studies (including cross-sectional, case–control and cohort studies); (3) primary outcome regarding the prevalence of MetS in patients with IBD or the association of MetS with IBD; and (4) original studies in the English language providing sufficient information to calculate the effect size. All studies were limited to those involving human subjects,and animal studies, case reports, review articles, redundant studies or studies that did not report specific outcome were excluded.
Data extraction and risk of bias assessment
Two researchers (YL and MZ) independently identified relevant literature by reading the titles, abstracts and full texts of the studies retrieved. The following information was subsequently extracted using a pre-established literature extraction table: author, journal, title, year of publication, contact information, country, study design, study population characteristics (participants, proportion of CD and UC, sample size, diagnosis criteria, general demographic information), clinical characteristics (duration, activity, severity, treatment), outcomes (prevalence, OR, risk ratio, risk factors), conclusion (association of MetS with IBD), etc.
Since all the eligible studies were observational, methodological quality was assessed using the Newcastle-Ottawa Scale (NOS) and an 11-item checklist recommended by the Agency for Healthcare Research and Quality (AHRQ).11 12 The NOS was used to evaluate the quality of cross-sectional or cohort studies, including three categories: selection (four items, one star for each item), comparability (one item, up to two stars) and outcome (three items, one star for each item). Thus, a study can be awarded up to a maximum of nine points. The quality of the study was classified as low (0–4 points), moderate (4–6 points) and high (7–9 points). The AHRQ checklist was employed to assess the risk of bias in cross-sectional studies based on 11 questions. An item was scored ‘0’ if the answer was ‘no’ or ‘unclear’; if the answer was ‘yes’, then the item was scored ‘1’. The quality of the study was assessed based on the total score. Overall, the results were divided into three levels: high quality (8–11 points), moderate quality (4–7 points) and low quality (0–3 points). Any discrepancies between two investigators were resolved by consulting a third reviewer (ZS).
Data synthesis and statistical analysis
Statistical analyses were performed using the packages (ie, meta and metafor) in R (V.4.2.1; R Foundation for Statistical Computing).13 The pooled prevalence of MetS among IBD was calculated as an aggregate mean, weighted by the sample size of each included study. The log-transformed proportions, logit-transformed proportions, arcsine transformed proportions and Freeman-Tukey double arcsine transformed proportions were used to stabilise the variance of individual studies. If the results were inconsistent, the Freeman-Tukey double arcsine transformation was preferred over other methods. Subsequently, unadjusted ORs were pooled from studies that had included a comparison group to give overall estimates of the association between MetS and IBD (UC or CD). All the values were estimated with 95% CI. Both the fixed-effect model and random-effects model were applied to estimate the pooled estimates. Given the conservativeness of results, the random-effects model proposed by DerSimonian-Laird (1986) was considered to be the primary method.14 Subgroup (stratified) analyses were performed according to IBD phenotypes (UC and CD), and sensitivity analyses were conducted by recalculating the pooled estimates after omitting studies of low quality. Furthermore, we narratively summarised data regarding risk factors for MetS among patients with IBD. Statistical heterogeneity was assessed using the inconsistency index (I²) and Cochrane Q statistic.15 The results were classified into three levels: low heterogeneity (I2 <25%), moderate heterogeneity (25%<I2<50%) and high heterogeneity (I2 >75%). We defined significant heterogeneity if the p value was <0.1 and I2 was >50%. Potential publication bias was evaluated by funnel plot and Egger’s test.16
Patient and public involvement
None.
Results
Literature search
A total of 3176 relevant records were initially identified. After the preliminary screening, 1499 articles were removed due to duplication. Based on the inspection of titles and abstracts, 85 potential studies were retrieved for further evaluation. After examining the full text, 11 of these publications met the predefined eligibility criteria and were included in our meta-analysis.6 8 17–25 The PRISMA flow diagram of the search strategy and study selection is illustrated in figure 1.
Flow chart of the meta-analysis. This flow chart, which shows the whole process of literature retrieval, screening, inclusion and exclusion, is based on PRISMA framework. IBD, inflammatory bowel disease; MetS, metabolic syndrome; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics
We found that all included studies investigated the prevalence of MetS in patients with IBD (rather than the prevalence of IBD in patients with MetS). All the studies were published after 2010, and six (55%) were cross-sectional in design. Three studies were conducted in North America, five in Europe and three in Asia. In total, 2501 patients with IBD were included in this study; 1678 (67.1%) had a diagnosis of CD and 823 (32.9%) had a diagnosis of UC. In most of the studies, IBD along with UC and CD was defined by international diagnostic criteria (eg, European Crohn′s and Colitis Organisation), and MetS was identified using the National Cholesterol Education Program Adult Treatment Panel III criteria. Of the 11 included studies, 1 (9.1%) comprised both the IBD group and the non-IBD comparison group, while 10 (90.9%) included only one disease cohort. Among these studies, one (9.1%) was limited to patients with UC, one (9.1%) to patients with CD and nine (81.8%) to patients with a mixed sample (ie, one that contained patients having both UC and CD). Four (44.4%) of the mixed-sample studies reported MetS prevalence separately by different IBD phenotypes (CD and UC). The general characteristics of the studies included are given in table 1.
Main characteristics of studies included in the meta-analysis
Risk of bias of included studies
Given the types of studies included, we used the NOS and AHRQ checklist to appraise the risk of bias for each study. However, some questions were not applicable. The majority of the studies scored well in terms of patient selection and outcome assessment, whereas one study was rated at high risk in that it did not report relevant information. Overall, the risk of bias of the included studies was moderate and acceptable. The results of the assessment are illustrated in figure 2 and online supplemental table 2.
Risk of bias of included studies using the 11-item checklist recommended by the Agency for Healthcare Research and Quality. A navy blue dot denotes low risk of bias, orange for unclear risk of bias and light green for high risk of bias.
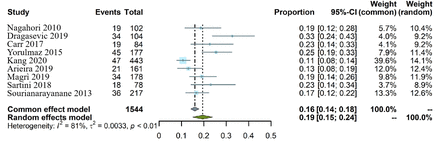
Overall prevalence of MetS among patients with IBD
We identified nine studies that reported available information regarding the prevalence of MetS among patients with IBD. Five of them were limited to analysis of the overall prevalence of comorbid MetS in patients with IBD, while the remaining four studies were subsequently pooled into subgroup analyses. A total of 273 MetS cases were detected among 1544 patients with IBD. Overall, the prevalence of comorbid MetS ranged from 10.6% to 32.7%. As a result, the pooled prevalence of MetS in IBD was estimated to be 19.4% (95% CI 15.1% to 23.8%). Since there was substantial statistical heterogeneity across the studies (I²=81.0%, Cochrane Q statistic=42.2, p<0.001), a random-effect model was used in our study. These unadjusted prevalence estimates and study heterogeneity are illustrated in a forest plot (figure 3). There was no evidence of publication bias according to Egger’s test (p=0.332), and the funnel plot is almost symmetrical (online supplemental figure 1). It is worth noting that these proportions were determined by type of design, source of subject, quality of study and method of outcome assessment. Therefore, we conducted further analyses. Sensitivity analyses revealed similar results (pooled estimate 20.7%; 95% CI 16.6% to 24.8%), and Kang et al’s study21 had the largest influence on the results (online supplemental figure 2). After excluding two studies of low quality and with ambiguous information,21 23 we found that the overall pooled prevalence was 21.9% (95% CI 18.0% to 25.8%), with moderate heterogeneity (I2=51.8%, Cochrane Q statistic=12.4, p=0.053). Overall, only one study reported the prevalence of MetS in non-IBD controls.6 MetS was more frequent in patients with IBD (32.7%) than in the non-IBD control group (13.3%), and there was a significant positive association between MetS and CD (p=0.01).
Forest plots for the overall pooled prevalence of comorbid metabolic syndrome among patients with inflammatory bowel disease.
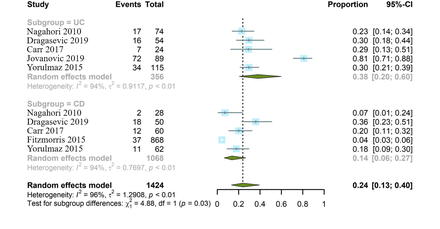
Stratified analyses of comorbid MetS between patients with UC and CD
Taking the subtype of IBD into account, we performed stratified analyses. In total, six included studies provided specific information regarding the prevalence of comorbid MetS in either UC or CD.6 8 17–20 All the studies were divided into two groups: 356 patients with UC (n=5 studies) in the UC analyses and 1068 patients with CD (n=5 studies) in the CD analyses. The pooled prevalence of comorbid MetS was 38.2% in UC (95% CI 20.4% to 59.9%) and 13.6% in CD (95% CI 6.4% to 26.7%). Strikingly, the aggregate estimate of MetS was significantly higher in UC than in CD (Cochran-Mantel-Haenszel χ2=4.88, p=0.03). Nevertheless, significant heterogeneity was observed (I2=94%, p<0.01). Detailed information is shown in figure 4. Sensitivity analyses by omitting two heterogeneous studies showed that MetS was more frequent in UC than in CD (27.7% vs 20.0%), with decreasing heterogeneity (I2=40.2%, p=0.11).18 19 However, no statistically significant difference (Cochran-Mantel-Haenszel χ2=1.64, p=0.2) was reached (online supplemental figure 3).
Stratified analyses by type of inflammatory bowel disease. The summary estimates were obtained using a random-effects model. The diamond data markers indicate the pooled proportion. CD, Crohn’s disease; UC, ulcerative colitis.
When we only included mixed-sample studies that reported comorbidity of MetS separately by different IBD phenotypes (n=4 studies), the meta-analysis demonstrated a negative association between MetS and UC compared with CD controls (pooled OR=1.52, 95% CI 0.96 to 2.41, p=0.073).6 8 17 20 Except for the study by Dragasevic et al6 which had an OR of 0.748, the remaining three studies reported an OR above 1.00. As shown in the forest plot (online supplemental figure 4), a low to moderate heterogeneity was detected (I2=35.9%, Cochrane Q statistic=4.68, p=0.197). Similarly, sensitivity analyses were conducted to investigate the stability of the results. We found that Dragasevic et al’s6 study had a significant impact on the results. After omitting Dragasevic et al’s study, the pooled estimate appreciably changed to become 2.11 (95% CI 1.19 to 3.74, p=0.01), which implies a risk approximately twice higher in UC than in CD (online supplemental figure 5). Although there was no evidence of statistical heterogeneity (I2=0%, Cochrane Q statistic=4.68, p=0.197), the number of studies that separately reported the outcome was small.
Risk factors for MetS among patients with IBD
There were four studies that specifically investigated relevant risk factors associated with MetS among patients with IBD.8 17–19 One of these was by Nagahori et al8 which found no statistical difference in gender, IBD phenotype, treatment, social history or health-related lifestyle between IBD patients with and without MetS. However, IBD patients with MetS were older than those without (50.2±15.0 vs 38.0±11.9, p=0.013). Moreover, age was an independent risk factor for MetS among patients with IBD in a multivariate logistic regression analysis (OR=1.064, 95% CI 1.017 to 1.114). The retrospective cohort study based on electronic healthcare record demonstrated that patients with IBD with concomitant MetS were statistically significantly older at the time of IBD diagnosis (p=0.005).17 In addition, IBD patients with MetS had overall higher prevalence of obesity, hypertension and diabetes or insulin resistance than IBD patients without MetS (p<0.001). Similarly, Fitzmorris et al18 from the USA reported that CD patients with MetS were older as compared with those without MetS (p<0.001). However, there was no statistically significant difference in gender, race or duration of disease between those two groups (p>0.05). Remarkably, after multivariate adjustment (eg, age, sex, race, duration of CD), patients with MetS had a CD-related hospitalisation rate twice than those without MetS (OR=1.91, 95% CI 1.12 to 3.26). Interestingly, the study by Jovanovic et al19 revealed similar results that patients with UC and MetS were significantly older compared with UC patients without MetS (p=0.001). Furthermore, UC patients with MetS reported higher values in cholesterol, triglycerides, low-density lipoprotein, interleukin 10 (IL-10) and galectin-3, compared with patients suffering from UC only. As a result, UC patients with MetS had lower Mayo endoscopic subscore (p=0.038) and Mayo clinical score (p=0.005), indicating that patients with UC and MetS have milder conditions. Overall, four studies identified that age was a statistically significant risk factor associated with the development of MetS. However, only one study further performed a multivariate analysis and only two satisfied the criteria. Given the limited number of studies, we failed to conduct a meta-analysis to elucidate the association between age and the incidence of MetS. Other variables (eg, obesity, diabetes) were also potential risk factors for the development of MetS among patients with IBD. Unfortunately, most of the included studies did not provide valuable data.
Discussion
Our study demonstrates the pooled prevalence of MetS in the IBD population from 11 studies, having a combined total of 2501 subjects. The present data reveal that MetS is not a rare complication among patients with IBD, as the pooled prevalence of MetS in IBD was 19.4% (95% CI 15.1% to 23.8%). To further understand the significance of the prevalence of comorbid MetS in IBD, we compared our result with external data reported by other investigators. It is reported that the prevalence of MetS in the general population ranged from 16.5% to 34.7%,26–33 and it tends to be more frequent in Western countries.34 A cross-sectional study from the USA evaluated the MetS prevalence among 17 048 adult participants, from 2011 to 2016, and showed a prevalence of 34.7% (95% CI 33.1% to 36.3%).26 The prevalence of MetS from our study appeared to be lower than that in the US adult population,26 27 while it was comparable to that in the Asia Pacific region and Middle East countries.28–33 Given the fact that the studies included in this meta-analysis were mainly from Europe, America and Asia, we suspect the prevalence of MetS in IBD was determined by region, age and method of outcome assessment. Therefore, the calculated comorbidity rate of MetS and IBD may not fully reflect its actual global prevalent status.
MetS develops as a result of progressive weight gain, fat mass accumulation and insulin resistance. MetS is a complex pathophysiological state that originates primarily from an imbalance of calorie intake and energy expenditure, genetic/epigenetic make-up of an individual, predominance of sedentary lifestyle, and other factors like quality and composition of food and composition of gut microbes. MetS is associated with a marked increase in risk of cardiovascular disease (CVD) and type 2 diabetes, possibly due to abdominal obesity, hyperglycaemia, dyslipidaemia and hypertension.34 35 A clear increase in the prevalence of MetS with ageing has been largely recognised; there are many commonalities in biochemical changes of the ageing process and MetS. According to our analysis, age might be a statistically significant risk factor involved in the association between MetS and IBD and so evaluation for MetS is needed in elderly patients with IBD.
Attention on comorbidity is crucial when managing patients with IBD because they can alter disease activity and extraintestinal manifestations, influence disease prognosis and influence pharmacological therapeutic effects. Both MetS and IBD are increasingly globally prevalent diseases. The pathogenesis and characteristics of the disease course of MetS in the IBD population are not entirely clear, and the pathogenesis of MetS in the IBD population may be more complex. Researchers have reported MetS and IBD share common pathophysiological features such as immune imbalance, chronic inflammation, disturbed secretion of adipokines and increased risk of CVD.3 36 Although our data did not show a close association of CVD risk in IBD with MetS, given the fact that MetS accelerates atherogenesis, eventually resulting in CVD, and that systemic inflammation can contribute to atherogenesis, an increased risk of CVD in patients with IBD and MetS cannot just be ignored.
The adipose tissue, and particularly the visceral adipose tissue (VAT), plays an important part in the pathophysiology of MetS. It is suggested that VAT may participate in chronic systemic inflammation of both MetS and IBD.3 37 VAT is composed of hypertrophic adipocytes that secrete abnormal levels of adipokines; for example, it may downregulate the synthesis of leptin, adiponectin and adipocytokines responsible for proinflammatory and anti-inflammatory effects.38 A lower level of serum and mesenteric adiponectin was observed in active CD, indicating adiponectin is associated with a defective regulation of anti-inflammatory pathways in CD pathogenesis.39 VAT also produces proinflammatory cytokines such as interleukin 6 (IL-6), tumour necrosis factor-α (TNF-α) and monocyte chemoattractant protein 1, leading to infiltration of M1 macrophages and causing low-grade chronic inflammation. M1 macrophages can also promote hepatic steatosis and adipogenesis.3 37 Reversely, the inflammation may also affect adipose tissue and disturb the adipokine secretion in MetS and IBD. It is reported that inflammation may induce dyslipidaemia through downregulation of lipoprotein lipase enzyme affected by the action of proinflammatory cytokines TNF-α, IL-6 and interferon-γ.40 In the included studies, some showed that IBD lipid profile was characterised by decreased total cholesterol and high-density lipoprotein (HDL) cholesterol. HDL performs many anti-inflammatory activities, which indicates that decrease in its level could not only be the effect but also the cause of intestinal chronic inflammation.41
As reported, gut dysbiosis is probably an additional factor that could alter the immune-metabolic state in IBD and MetS.34 Inflammation and the gut microbiome can trigger intestinal barrier dysfunction, while in IBD the disruption of the gut barrier allows microbe infiltration into the submucosa, which enhances the probability that gut-derived metabolites infiltrate from the gut to the liver and pancreas. Therefore, gut microbial dysbiosis may be one of the potential mechanisms contributing to comorbidity of MetS and IBD via increased intestinal permeability.2 A recent study reported gut virome changes have an association with MetS and exhibit decreased richness and diversity, providing a starting point to studies of phage effects on gut bacteria and the role that this plays in MetS.42 Akkermansia muciniphila is a Gram-negative and mucin-degrading bacterium which is highly abundant in the gut microbiota. Reduced levels of A. muciniphila have been observed in patients with IBD (mainly UC) and metabolic disorders, which suggests it may have potential anti-inflammatory properties linked to impaired gut–barrier integrity.43 A recent study shows that pasteurisation of A. muciniphila enhances the bacterium’s ability to reduce fat mass and MetS in mice with diet-induced obesity, which may be a strategy to fight against obesity and IBD.44 It is reported that intestinal microbiota protects against the development of MetS by inducingT helper cell 17 (Th17) and regulating lipid absorption across the intestinal epithelium. However, high-fat, high-sugar diet will promote metabolic disease by depleting Th17-inducing microbes. The findings highlight an interaction between diet, microbiota and intestinal immunity in metabolic disorders.45
In our current meta-analysis, MetS prevalence is found to be significantly lower in CD than in UC (OR=2.11, 95% CI 1.19 to 3.74, p=0.01). Considering the average age of patients with UC is older in these studies, age may be a confounding factor. A study reported that the prevalence of MetS in UC reached 81% and the average age of the patients was 50 (21–80). The study also indicated that patients with MetS have milder forms of UC, with higher serum level of immunosuppressive cytokine IL-10 and faecal content of galectin-3, and proposed that the presence of MetS may limit the inflammatory process and subsequent tissue damage in UC possibly by deviating local inflammatory response towards enhanced participation of immunosuppressive cells and molecules.19 CD and UC have been postulated to involve different immunological backgrounds. The inflammation in UC primarily involves the colonic mucosa. In contrast, features of CD are transmural inflammation affecting all layers of the intestinal wall and mesenteric lymph nodes and chronic non-caseating granulomatous inflammation.46 Underweight is more frequently observed in patients with CD, as lack of proper gut function reduces nutrient absorption. CD is often accompanied by malnutrition and thus might not present the classic symptoms of MetS.47 The presence of MetS has been shown to increase the rate of hospitalisations in patients with CD.36 37 A study focused on patients with CD showed a 4.3% comorbidity rate of MetS and presented a CD-related hospitalisation rate twice of those who did not have MetS. MetS is supposed to exacerbate mesenteric inflammation and may trigger symptomatic CD, which may be associated with risk factors including high triglycerides, low HDL cholesterol and diabetes mellitus.18 What makes sense is that a healthy lifestyle should always be advised and promoted in IBD care to prevent metabolic disorders. In terms of diet, nutritional and metabolic interventions to avoid the development of metabolic complications associated with an unbalanced diet are necessary. Consumption of a Western dietary pattern, meat and fried foods promotes the incidence of MetS.48 The PREDIMED trial (Prevención con Dieta Mediterránea) and other studies had evidenced a beneficial role of traditional Mediterranean diet (higher in monounsaturated fatty acids) in preventing both MetS and IBD.49 Second, it is suggested that patients with IBD who smoke quit to prevent the risks of long-term extradigestive effects. Especially in CD, smoking is reported to increase the risk of hospitalisation.36 In addition, physical activity is encouraged as exercise is a key component of energy expenditure and energy balance.
To the best of our knowledge, no epidemiological meta-analysis has yet systematically investigated the association between MetS and IBD. Our study presents the most comprehensive meta-analysis of the prevalence of MetS in patients with IBD. However, this review has several limitations. Above all, given the fact that most of the studies included in our study were cross-sectional in design, some potential confounding factors could lead to bias in the association between MetS and IBD. Additionally, most studies did not establish a control group of patients without IBD. Another weakness of this meta-analysis is the lack of calculation of the OR of MetS compared with IBD. Therefore, we cannot confirm whether MetS is more common in IBD than in the general population. Besides, as the number of studies included is small, the MetS prevalence estimates may be unstable due to the small sample size of some studies. Meanwhile, region, ethnicity, age and different diagnostic criteria for MetS may also be sources of heterogeneity, and publication bias may limit the generalisability of the results.
The principal conclusion of this meta-analysis is that MetS is not uncommon in patients with IBD, especially in UC and in older patients. Additional studies are required to determine more precisely the prevalence of MetS in the general population of individuals with IBD. Such studies could also further investigate potential risk factors so that both adjusted and unadjusted prevalence of IBD with MetS can be calculated. Collectively, early detection of MetS can be expected to benefit patients with IBD and lead to better disease outcomes. Application of prevention measures for diabetes and CVD in patients with MetS and IBD may be required to improve their long-term prognosis, particularly in older patients. Mechanistic studies are also needed to further explore the potential relationships between MetS and IBD.
Supplemental material
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
No ethical approval was required.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
ZS, MZ and YL are joint first authors.
ZS, MZ and YL contributed equally.
Contributors Concept and design: LZ, HS and ZS. Literature retrieval and study selection: YLi and MZ. Data extraction and collection: YLi, CG and ZS. Analysis and interpretation of data: ZS, YLu and LZ. Supervision and validation: CG, HS and LZ. Drafting of the original manuscript: MZ and ZS. All authors have approved the final draft of the manuscript. ZS, MZ, YLi and LZ have full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. LZ is guarantor.
Funding This work was supported by the National Key Research and Development Program of China (grant number 2017YFC1700104) and the Jiangsu Province Traditional Chinese Medicine Science and Technology Development Project (grant number ZT202103 and YB2020008).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.