Article Text
Abstract
Introduction Early sepsis treatment in the emergency department (ED) is crucial to improve patient survival. Despite international promulgation, the uptake of the Surviving Sepsis Campaign (SSC) Hour-1 Bundle (lactate measurement, blood culture, broad-spectrum antibiotics, 30 mL/kg crystalloid for hypotension/lactate ≥4 mmol/L and vasopressors for hypotension during/after fluid resuscitation within 1 hour of sepsis recognition) is low across healthcare settings. Delays in sepsis recognition and a lack of high-quality evidence hinder its implementation. We propose a novel sepsis care model (National Early Warning Score, NEWS-1 care), in which the SSC Hour-1 Bundle is triggered objectively by a high NEWS-2 (≥5). This study aims to determine the feasibility of a full-scale type 1 hybrid effectiveness-implementation trial on the NEWS-1 care in multiple EDs.
Methods and analysis We will conduct a pilot type 1 hybrid trial and prospectively recruit 200 patients from 4 public EDs in Hong Kong cluster randomised in a stepped wedge design over 10 months. All study sites will start with an initial period of standard care and switch in random order at 2-month intervals to the NEWS-1 care unidirectionally. The implementation evaluation will employ mixed methods guided by the Reach, Effectiveness, Adoption, Implementation and Maintenance framework, which includes qualitative and quantitative data from focus group interviews, staff survey and clinical record reviews. We will analyse the 14 feasibility outcomes as progression criteria to a full-scale trial, including trial acceptability to patients and staff, patient and staff recruitment rates, accuracy of sepsis screening, protocol adherence, accessibility to follow-up data, safety and preliminary clinical impacts of the NEWS1 care, using descriptive statistics.
Ethics and dissemination The institutional review boards of all study sites approved this study. This study will establish the feasibility of a full-scale hybrid trial. We will disseminate the findings through peer-reviewed publications, conference presentations and educational activities.
Trial registration number NCT05731349.
- accident & emergency medicine
- infectious diseases
- qualitative research
- randomized controlled trial
- feasibility studies
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- accident & emergency medicine
- infectious diseases
- qualitative research
- randomized controlled trial
- feasibility studies
STRENGTHS AND LIMITATIONS OF THIS STUDY
The National Early Warning Score (NEWS-1) care is a novel sepsis care model that applies the Surviving Sepsis Campaign Hour-1 Bundle to adult patients with an infection and a high NEWS-2 (≥5) in emergency departments (EDs).
This study will provide data that inform whether it is feasible to conduct a full-scale type 1 hybrid trial on the effectiveness and implementation of the NEWS-1 care in multiple EDs in Hong Kong.
This study also evaluates the barriers to and facilitators of the implementation of the NEWS-1 care in the ED, which is important in tailoring implementation strategies to suit local contexts.
Due to the stepped wedge study design, the need for implementation before crossing over from standard care to NEWS-1 care, and differences in clinical interventions, allocation concealment of the cross-over date and blinding of the patients, clinicians and assessors are not possible.
Introduction
Sepsis is a medical emergency with a substantial impact on public health worldwide, not only because it is life-threatening and debilitating, but also because it is costly to treat.1–3 In Hong Kong, 39% of hospitalised adult patients with suspected infection developed sepsis, of whom 20% died.4 The burden of sepsis continues to rise in the face of an ageing population; increasing burden of chronic disease; growing use of immunosuppressive therapy, transplantation, chemotherapy, invasive procedures and devices; and the emergence of novel pathogens and multidrug-resistant infections.5 A significant proportion of patients with sepsis are admitted to the hospital through the emergency department (ED), where appropriate intervention might have a significant impact on the downstream clinical course.
Early diagnosis, coupled with prompt correction of physiological derangements, antimicrobial therapy and control of the source of infection, are the cornerstones of sepsis care. Since 2004, the Surviving Sepsis Campaign (SSC) has periodically released guidelines on sepsis management.6 In 2005, the SSC introduced the idea of ‘sepsis bundles’, which mandated completion of core interventions, such as administration of antibiotics, within fixed time frames.7 The 2018 SSC Hour-1 Bundle requires initiation of five interventions within 1 hour of sepsis recognition (lactate measurement, blood cultures before antibiotics, broad-spectrum antibiotics, 30 mL/kg crystalloid for hypotension or lactate level ≥4 mmol/L and vasopressors for hypotension during or after fluid resuscitation).8 Despite international endorsement and promulgation, the uptake of sepsis care bundles and their individual components remains low and inconsistent across healthcare settings,9–13 leading to a variable standard of care and suboptimal patient outcome in many cases.
Multiple barriers exist in the ED that hinder the uptake of sepsis care bundles.14 First, sepsis recognition is more difficult in EDs than on wards or in intensive care units (ICUs).15 16 The Sepsis-3 definition of sepsis requires laboratory tests to show evidence of organ injury with an acute change in the Sequential Organ Failure Assessment (SOFA) score ≥2.17 These tests generally have a turnaround time of 1–2 hours, causing a delay in sepsis diagnosis. The quick SOFA (qSOFA) score (based on three clinical criteria: respiratory rate (RR) >21/min; systolic blood pressure (SBP) ≤100 mm Hg and altered mental status) has been introduced to identify high-risk patients outside ICUs but is not suitable for ED sepsis screening because of its low sensitivity.18 Second, the SSC Hour-1 Bundle remains a subject of debate with criticism of potential overdiagnosis and overtreatment.19 Evidence from observational studies20–23 supports a reduction in sepsis mortality and costs with the bundle care approach but high-quality evidence from randomised controlled trials is lacking. Third, the delivery of the bundle components involves multiple interdependent tasks for doctors, nurses and other carers, which are frequently interrupted by other competing demands in the ED. Execution is prone to coordination and operation failures.24 A lack of additional staff and specialised settings for the procedures also make translation into daily clinical practice challenging.15 16
National Early Warning Score-1 care
Current evidence suggests that the NEWS is superior to the qSOFA in recognising sepsis and predicting mortality in the ED.25–30 The NEWS can be calculated based on six physiological parameters routinely measured at triage: RR, oxygen saturation, SBP, pulse rate, level of consciousness or new confusion and temperature. The updated version, the NEWS2, has been endorsed by National Health Service (NHS) England and NHS Improvement as the routine early-warning system.31 A NEWS-2≥5 flags a serious risk of clinical deterioration and triggers urgent assessment and intervention.
We propose a novel sepsis care model in which the SSC Hour-1 Bundle is triggered objectively by a high NEWS2 score (≥5). This care model, called the NEWS-1 care, has two theoretical advantages over standard care: (1) early initiation of the SSC-1 bundle in the ED based on clinical parameters to avoid delays and (2) standardisation of sepsis care based on objective criteria. We are planning to conduct a multicentre, hybrid type 1 effectiveness-implementation trial to evaluate the effectiveness of NEWS-1 care while gathering information on the barriers to and facilitators of its implementation in the ED. Given the complexity of the study design, we will first conduct a pilot study to determine the feasibility of a full-scale trial.
Study aims and objectives
The aim of this pilot study is to determine the feasibility of conducting a fully powered type 1 hybrid trial on the NEWS-1 care in multiple EDs in Hong Kong with the following objectives:
To assess the feasibility of emergency staff in multiple centres to screen, recognise and recruit patients with suspected sepsis.
To assess the feasibility of emergency staff in multiple centres to execute trial procedures.
To assess the feasibility of trial methods to decide whether a fully powered trial should be undertaken to determine the safety and effectiveness of the intervention.
To assess the feasibility of research staff to conduct the implementation evaluation.
Methods and analysis
This pilot study will replicate the planned full-scale hybrid trial in miniature to evaluate its feasibility based on 14 predefined progression criteria. The target full-scale trial will have two components: an effectiveness component and an implementation component. This pilot study will be conducted in four public EDs under the Hospital Authority in Hong Kong: Queen Mary Hospital, Pamela Youde Nethersole Eastern Hospital, Prince of Wales Hospital and Tuen Mun Hospital over 10 months from June 2023 to March 2024. Online supplemental file 1 summarises the characteristics of these four study sites. In essence, none of these sites has routinely used the NEWS2 or SSC Hour-1 Bundle before the trial.
Supplemental material
Effectiveness trial feasibility
To evaluate the feasibility of the effectiveness component of the target full-scale trial, we will prospectively recruit 200 patients (50 patients per site) from the 4 study sites that are cluster randomised in a stepped wedge fashion in this pilot study. The stepped wedge design combines elements of a standard cluster-randomised design and a before–after design.32 The unit of randomisation is each individual ED. It is considered optimal for evaluating the effectiveness of the NEWS-1 care in the full-scale trial because: (1) the conventional randomisation procedure can delay sepsis care and (2) once healthcare professionals have been trained to use NEWS2 and to link the score to the SSC Hour-1 Bundle, it is not possible to ‘untrain’ them. Concurrently offering standard care and NEWS-1 care in a study site may result in the adoption of the SSC Hour-1 bundle by many patients in the standard care group, causing contamination. Given the short exposure time to the intervention, the carry-over effect and within-cluster contamination are negligible.32 Contamination across the study sites is also expected to be minimal because only one or two higher resident trainees will rotate between the sites during the study period and their clinical practice is supervised by local specialists in emergency medicine.
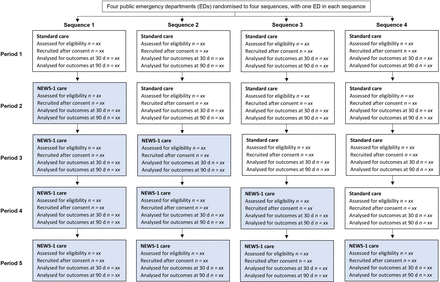
Allocation concealment from the cluster is achieved by performing randomisation after recruitment of all four study sites. Randomisation has been performed using an allocation sequence based on computer-generated random numbers at a single time point before the trial starts. All study sites start with an initial period of standard care and then switch in random order at 2-month intervals to the NEWS-1 care in a unidirectional fashion until all EDs have crossed over (figure 1). Recruitment will continue in all study sites until the end of the trial. Given the need for implementation strategies before the cross-over date in the study sites, allocation concealment of the cross-over date is not possible. Blinding of the patient participants, clinicians and assessors are not possible due to differences in treatment offered at the ED.
The stepped wedge design for the NEWS-1 pilot randomised controlled trial. ED, emergency department; NEWS, National Early Warning Score.
Patient participants
Inclusion criteria
ED patients aged ≥18 years who fulfil all of the following criteria:
A clinical diagnosis of infection made by the treating emergency physicians.
Require hospital admission.
A NEWS2≥5.
Exclusion criteria
Patients will be excluded if they meet any one of the following criteria:
Age <18 years.
Currently pregnant.
Postchemotherapy fever, for which existing ED protocols for early antibiotics apply.
An advanced directive with a ceiling-of-care.
Refusal of consent/pre-existing mental illness rendering consent impossible.
Refusal of hospitalisation.
Patient participant recruitment
Full-time trained research nurses are deployed in the study sites (9-hour shift per day) to screen for eligible patients and to recruit patient participants throughout the study period. For adult patients with a clinical diagnosis of infection, the research nurses will calculate the NEWS2 based on the worst vital signs during the ED stay. If a vital sign is not recorded, the nurse will approach the patient and measure it after obtaining verbal consent. A screening log of all patient encounters will be maintained.
Written informed consent will be obtained from all patient participants or their family members after an explanation of the details of the study, including the rationale, benefits and risks of participation. During the standard care period, consent is sought for data use. After the cross-over date, consent is sought for NEWS-1 care. For eligible patients with altered mental status judged to be related to sepsis, efforts will initially be made to obtain written consent from their legal guardians, followed by retrospective consent when the patient regains sufficient mental fitness to give informed consent. After obtaining informed consent, each recruited participant will be assigned a study code, which is confidentially linked to patient identifiable number for data retrieval and follow-up.
Standard care
During the standard care period, emergency physicians will assess the patients based on history, physical examination, laboratory tests and imaging studies, and will offer treatment based on clinical expertise, intuition and local or international guidelines. The attending emergency physicians may offer elements of the SSC Hour-1 Bundle based on clinical judgement.
NEWS-1 care
NEWS-1 care consists of routine clinical assessment plus calculation of the NEWS2 in all adult patients with an infection who will be admitted to the hospital and providing SSC Hour-1 Bundle care when the NEWS2≥5 within 1 hour of sepsis recognition. Time ‘zero’ is defined as when sepsis is recognised (ie, the time when a clinical diagnosis of infection is made AND a NEWS2≥5). The time of clinical diagnosis of infection is captured automatically by the standard electronic medical record system across all the study sites when the attending emergency physicians enter an infection-related diagnostic code to proceed with the hospital admission procedure. Likewise, the time of decision on hospital admission and vital sign measurement is captured by the electronic medical record system and the patient participant screening log in a real-time manner.
The Bundle care includes:
Measure lactate level and remeasure if initial lactate >2 mmol/L.
Obtain blood cultures before administering antibiotics.
Administer parental broad-spectrum antibiotics.
Begin rapid administration of 30 mL/kg crystalloid for hypotension or lactate level ≥4 mmol/L.
Apply vasopressors if the patient remains hypotensive during or after fluid resuscitation to maintain the mean arterial blood pressure ≥65 mm Hg.
The treating physicians can exercise clinical judgement to avoid fluid overload in susceptible patients (eg, those with heart or renal failure). Local hospital guidelines will be followed for the use of vasopressor and in the choice of empirical antibiotics.33
Since sepsis care is a dynamic and continuing process, the focus will be on initiating all components of the SSC Hour-1 Bundle in the ED, as clinically appropriate, without specifying the duration of therapy or the length of ED stay. During both the standard care and NEWS-1 care periods, treatment decisions other than the components specified in the SSC Hour-1 Bundle—such as laboratory investigations, imaging studies, other ED treatment and patient disposition—will be determined by the treating emergency physicians. After hospital admission, all subsequent decisions about the level of care, monitoring, investigations and treatment will be determined by the clinical team in the receiving ward or ICU.
Patient follow-up
We will follow up the clinical progress of all recruited patient participants until 90 days after enrolment or death. Data collection will start at the time of enrolment using a standard form. The research nurses will trace all laboratory and microbiological results and patient outcomes by reviewing the electronic medical records. For patients who are discharged or transferred to other hospitals before day 30 or day 90, research nurses will conduct telephone follow-up. The patient flow diagram is shown in figure 2.
Cluster and patient participant flow chart in the NEWS-1 pilot stepped wedge randomised controlled trial. NEWS, National Early Warning Score.
Preliminary clinical impacts
We will review the preliminary clinical impacts of the NEWS-1 care, including all-cause in-hospital, 30-day and 90-day mortality; sepsis-related in-hospital mortality; ICU admission; ventilator-free days; the need for renal replacement therapy; the total ED, ICU and ward length of stay; and the time to surgery for cases who receive a surgery during hospitalisation, up to day 30 post-enrolment unless otherwise stated.
Safety issue and monitoring
The risks to the patient participants are minimal since the components in the SSC Hour-1 Bundle are standard hospital therapies, with the exception that they are ‘front-loaded’ to the patients in the ED before hospital admission. We have set up an independent data monitoring committee (DMC) comprising three clinicians (one emergency physician, one infectious disease expert and one critical care physician) not involved in the study, to monitor the occurrence of adverse events in both arms every 3 months, including allergic reaction to antibiotics, new onset of Clostridium difficile infection up to 4 weeks of index hospital discharge, fluid overload, tachyarrhythmias and acute bowel or limb ischaemia attributed to ED inotrope use. The DMC will decide whether to terminate the study or to allow the study to proceed as planned with or without protocol amendment.
Implementation feasibility
Conceptual model and implementation strategies
Implementation strategies commonly reported to drive healthcare professional behavioural change in sepsis care include staff education, engagement and motivation; enrolment of senior clinical staff and clinical champions; department sepsis protocol; provision of prompts for behaviour and equipment required for task completion; improving staff communication; continued individual staff feedback and rewarding desired behaviour.34–38 In the literature, the number and type of implementation strategies do not have a consistent relationship with antibiotic administration time and no single strategy is clearly superior.39 Implementation of both educational and programme change programmes was associated with a higher bundle compliance and a greater mortality reduction compared with either programme alone.40
The implementation blueprint of our study is devised according to the Theoretical Domains Framework (TDF) in behavioural theory that identifies 14 theoretical domains, ranging from ‘knowledge’ to ‘social influences’ and ‘environmental context and resources’ that are relevant to healthcare professional behavioural change in sepsis care,41–44 as well as reported implementation strategies that are applicable in our local context. We follow the recommendations of the Workgroup for Intervention Development and Evaluation Research45 and Proctor et al46 in specifying each implementation strategy targeting staff behavioural change in seven domains: the actors involved, actions undertaken, action targets, timing or temporarily, dose, implementation outcomes and theoretical justification. To facilitate future replication, implementation and evidence synthesis, we use the Behavioural Change Technique (BCT) Taxonomy (V.1), an international taxonomic classification system with 93 distinct and consensually agreed active components of behaviour change interventions,47 in reporting BCTs. The description of implementation strategy is further supplemented by the taxonomy proposed by Powell et al48 and Mazza et al,49 which is based on the Cochrane Effective Practice and Organisation of Care checklist.50 Table 1 summarises the details of activities that are mapped to BCTs and TDFs to overcome operational and system barriers and to facilitate implementation when each study site is switching from standard care to NEWS-1 care.
Intervention content and mechanisms of action based on behavioural change techniques
Implementation evaluation
The implementation evaluation will employ mixed methods guided by the five elements of the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework,51 including reach (the uptake of the NEWS-1 care and the extent of ED staff participation), effectiveness (impact of the NEWS-1 care on patient outcomes), adoption (organisation support for the NEWS-1 care), implementation (delivery of the NEWS-1 care) and maintenance (long-term use of the NEWS-1 care). We will collect qualitative and quantitative data concurrently at multiple levels and analyse them separately to assess different elements of implementation and to obtain a more in-depth understanding of the observed pattern of implementation. Table 2 summarises the evaluation methods and data sources mapped with individual elements of the RE-AIM framework.
The RE-AIM evaluation dimensions for implementation of the NEWS-1 care at multiple levels
Two rounds of focus group interviews will be conducted: the first before trial commencement (from March to May 2023) and the second after the end of the enrolment period (T2). In each study site, a minimum initial stratified sample of six participants (one consultant/associate consultant, two junior doctors, one senior nurse of the rank advanced practice nurse or above and two junior nurses) will be invited. Purposive sampling will be used to recruit potentially eligible participants identified by the site investigators and executives. They will be approached either by email or in person over 6 weeks. Sampling will continue until data saturation is reached. In total, 24–30 participants will be invited to participate voluntarily in each of the two rounds of interviews. The number of invited participants who refuse to take part will be reported.
After obtaining written consent, the ED staff participants will be interviewed in small focus groups for up to 90 min with a semistructured interview guide (online supplemental file 2), which is developed based on TDF and our previous study results.13 The focus group interviews will explore the barriers to and facilitators of implementing the NEWS-1 care in local ED settings, the overall experience of the staff involved and ‘how’ and ‘why’ the implementation works or does not work. All interviews will be conducted by the principal investigator (a male emergency clinician researcher with qualitative research training) in the presence of a research nurse in a quiet private room in the hospital and audiorecorded. Verbatim transcription will be performed anonymously. The interviewees will be invited to review the transcripts for accuracy. Transcripts will then be analysed and coded in local language. The findings of focus group interviews at T1 will be used to refine the implementation strategy described in table 1 and the questions in the staff survey.
Supplemental material
A voluntary, anonymous staff survey will be conducted to assess the acceptability of the study to ED staff and to obtain a broader perspective on implementation. The survey will include questions with dichotomous responses (eg, do you agree to take part in this study?—Yes/No), five-point Likert-type scales and open-ended questions to assess participants’ awareness, knowledge and belief in sepsis care, and the importance of barriers and facilitators identified in focus group interviews (online supplemental file 3). The survey will be distributed to all emergency doctors and nurses at the study sites electronically and during meetings over 8 weeks.
Supplemental material
Evaluation of feasibility outcomes
The primary outcome is the feasibility of a full-scale type 1 hybrid trial on the NEWS-1 care in multiple EDs in Hong Kong, which will be determined based on the 14 predefined progression criteria. Table 3 shows the 14 predefined criteria mapped to individual study objectives and data sources. If a progression criterion is within 5% below the target, we will review the reasons and consider protocol modification. If a progression criterion is within 10% below the target, we will critically review the protocol and consider major amendments. If a progression criterion is more than 10% below the target, we will consider whether progression to a full trial is appropriate. We will not proceed to a full-scale trial if there is clear evidence of significant harm (safety criterion) or effect (equipoise criterion). As for the other progression criteria, no weighing will be added during evaluation and they will be considered collectively as we conclude the study.
Evaluation of progression criteria
Data processing and analysis
Quantitative data
The analysis of quantitative data will mainly be descriptive. We will report the number of participants at different stages of recruitment and follow-ups and their clinical characteristics, as well as whether each progression criterion is met. Missing values will not be imputed. We will report continuous variables as means and SD, or medians and IQRs, and categorical variables as proportions, as appropriate. Preliminary clinical impacts will be reported with estimates of the 95% CIs, but p values will not be reported because the study is not powered for testing hypothesis about effectiveness. In addition, we will describe the distribution of demographic characteristics, comorbidities and clinical severity of the patients recruited before and after the cross-over date to identify factors that might require adjustment in the full-scale trial. We will use the Statistical Package for the Social Sciences for Windows V.27.0 (IBM) or R V.4.2.2 for statistical analysis.
Qualitative data
For qualitative data, we will use grounded theory methodology and conduct thematic analysis with NVivo V.11 software (QSR International, Doncaster, Victoria, Australia). Qualitative data will be subjected to constant comparative analyses of responses across individuals to determine the core categories. The analytical process will be based on immersion in the data and repeated sorting, coding and comparison. After systematic comparisons of the categories, the properties and dimensions of the studied concepts will be developed from the interview data.52 53 In addition, selective sampling of the literature will be used to add completeness to the theoretical description. The principal investigator and a research assistant will perform coding independently to increase the validity of the theoretic constructs, with disputes resolved by repeated discussion, recoding and expert consultation.
Patient and public involvement
Given the pilot stage of this study, we did not involve patients or the public in developing the research questions, study design, choice of outcome measures and participant recruitment.
Ethics, data sharing and dissemination
We have obtained research ethical approval from the institutional review boards of all study sites (UW 22-094, HKECREC-2022-003, NTWC/REC/22011 and 2022.039-T). The study will be conducted in full compliance with the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (NCT05731349) on 16 February 2023.
Deidentified study data will be available on reasonable request 9 months after publication of the main results, subject to the additional research ethics approval on data sharing. Requests for data should be sent to lampkrex@hku.hk.
We will report the study findings according to the Standards for Reporting Implementation Studies Statement,54 Consolidated Standards of Reporting Trials (CONSORT) extension for the stepped wedge cluster randomised trial,55 CONSORT 2010 statement: extension to randomised pilot and feasibility trials56 and Consolidated Criteria for Reporting Qualitative Research.57 We will disseminate the results through peer-reviewed publications, conference presentations and educational activities with the public and professional bodies.
Discussion
The NEWS-1 TRIPS will provide real-world data from multiple sources to inform whether a full-scale type 1 hybrid trial is feasible in the ED setting. It will also provide useful data on possible secular trends over time due to factors external to the study, time-by-treatment interactions, within-period and between-period intraclass correlation coefficients, and differences in the demographic and clinical characteristics of patient participants recruited before and after the cross-over date, which are important confounding factors that require statistical adjustment in future full-scale trials. Information gathered from the implementation evaluation will help us improve and tailor implementation strategies based on the barriers and facilitators identified across different study sites.
The authors are aware that the latest SSC 2021 guidelines no longer recommend the SSC-Hour 1 Bundle for all patients with an infection. However, for adult patients with possible septic shock or a high likelihood for sepsis, the SSC guidelines still strongly recommend administering antimicrobials immediately, ideally within 1 hour of recognition.58 We believe that adult patients with an infection and a high NEWS2≥5 need prompt resuscitation. It is exactly the lack of high-quality evidence for the SSC Hour-1 bundle in this patient group that warrants an RCT to fill the current knowledge gaps.
There are several inherent limitations of the stepped wedge study design. We attempt to avoid selection bias of the cluster by performing randomisation at a single time point after all study sites have consented to participate. However, we cannot conceal the allocation of the cross-over date because of the need for implementation. The recruitment bias caused by recruiting staff foreknowledge of treatment assignment would be minimal because of the well-defined patient participant recruitment criteria. Blinding is also not possible because of the differences in clinical intervention in the standard care and NEWS-1 periods. Our study findings can be generalised to EDs where both the NEWS2 and SSC Hour-1 bundle are not routinely used. In EDs where NEWS2 is routinely calculated, the study results will be useful in improving sepsis care.
Ethics statements
Patient consent for publication
Acknowledgments
We wish to thank the Health and Medical Research Fund in Hong Kong for supporting this research.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors RPKL and THR conceptualised the study and acquired funding. RPKL, THR, KKCH, CTL, WSK, PYTN, CHC, TCT, MSHT and CAG supervise and recruit patients in the study sites. THR, CAG and SS provide supervision of the study. SS reviews and interpret microbiology results of the recruited patients and provides expert advice. RPKL and WWTL analyse qualitative data. RPKL and EHYL analyse quantitative data. All authors have read and approved the final manuscript.
Funding This study is supported by the Heath and Medical Research Fund of the Food and Health Bureau of the Hong Kong Special Administrative Region (Grant No. 19201161).
Disclaimer The content of this manuscript does not represent the views of the funding body.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.