Article Text
Abstract
Objectives To evaluate the diagnostic performance of urine HIV antibody rapid test kits in screening diverse populations and to analyse subjects’ willingness regarding reagent types, purchase channels, acceptable prices, and self-testing.
Designs Diagnostic accuracy studies
Participants A total of 2606 valid and eligible samples were collected in the study, including 202 samples from female sex workers (FSWs), 304 persons with injection drug use (IDU), 1000 pregnant women (PW), 100 subjects undergoing voluntary HIV counselling and testing (VCT) and 1000 students in higher education schools or colleges (STUs). Subjects should simultaneously meet the following inclusion criteria: (1) being at least 18 years old and in full civil capacity, (2) signing an informed consent form and (3) providing truthful identifying information to ensure that the subjects and their samples are unique.
Results The sensitivity, specificity and area under the curve (AUC) of the urine HIV-1 antibody rapid test kits were 92.16%, 99.92% and 0.960 (95% CI: 0.952 to 0.968, p<0.001), respectively, among 2606 samples collected during on-site screenings. The kits showed good diagnostic performance in persons with IDU (AUC, 1.000; 95% CI, 1.000 to 1.000, p<0.001), PW (AUC, 0.999; 95% CI, 0.999 to 1.000, p<0.001) and FSWs (AUC, 1.000; 95% CI, 1.000 to 1.000, p<0.001). The AUC of the urine reagent kits in subjects undergoing VCT was 0.941 (95% CI: 0.876 to 0.978, p<0.001). The ‘acceptable price’ had the greatest influence on STUs (Pi=1.000) and PW (Pi=1.000), the ‘purchase channel’ had the greatest influence on subjects undergoing VCT (Pi=1.000) and persons with IDU (Pi=1.000) and the ‘reagent types’ had the greatest influence on FSWs (Pi=1.000).
Conclusions The rapid urine test kits showed good diagnostic validity in practical applications, despite a few cases involving misdiagnosis and underdiagnosis.
- HIV & AIDS
- public health
- sensitivity and specificity
Data availability statement
Data are available upon reasonable request. The original database for this study contains private information about the study participants. For non-commercial use and reasonable purposes, anonymised data of the current work can be obtained from the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study has evaluated the diagnostic validity of urine HIV-1 rapid test kits in screening both the general population and high-risk populations.
Cluster analysis provides a clear profile of the main concerns and selection preferences of the different populations when they choose HIV test reagents.
No positive samples were found among the students, and therefore, receiver operator characteristic curves could not be plotted for this subgroup.
Introduction
The prevalence of HIV/AIDS varies widely across China.1 2 Guangxi Zhuang Autonomous Region, the only minority region in southern China, is a serious HIV/AIDS hotspot; in the past decade, this region had a much higher HIV/AIDS prevalence than any other Chinese coastal or inland province.3 4 Therefore, the public health administration in Guangxi is attempting to expand the scale of HIV screening to diagnose HIV-infected patients at an early stage and provide highly active antiretroviral therapy (HAART) promptly to reduce HIV/AIDS mortality and transmission,5 6 especially in high-risk populations.7
With the cost reduction, urine HIV antibody testing is gradually gaining attention and acceptance by public health policymakers, health institutions and the general public due to its advantages of being convenient, noninvasive, safe8–10 and reliable.11–14 However, these urine HIV antibody reagents required that urine samples be transported to the laboratory for centralised testing because of methodological limitations, which limits their convenience of application.
A urine HIV-1 antibody rapid test reagent with colloidal gold method was granted marketing approval by the China Food and Drug Administration in 2019. This reagent can present the results within 15 min, and all operations can be completed on site. Due to the advantages of non-invasiveness, convenience and rapidity, the Guangxi health department is very interested in this reagent and believes that adopting it may help to further increase the acceptance of the population to HIV screening. It is worth noting that although some studies have evaluated the diagnostic performance of urine HIV-1 antibody rapid test kits using standard samples under controlled laboratory conditions, no studies have yet reported on their diagnostic performance in practical applications and the acceptance of different populations; therefore, an adequate scientific basis for the application of urine rapid test kits for HIV screening has not been provided for public health authorities in high-prevalence areas.
This study, based on a special study of the Chinese National Science and Technology Major Project (NSTMP) for infectious diseases, aimed to evaluate the diagnostic performance of urine HIV-1 antibody rapid test reagents in a practical screening setting and to preliminarily analyse the willingness of subjects regarding the types of reagents, purchase channels and acceptable prices to provide a valuable scientific basis for the application of urine HIV antibody rapid test reagents for screening.
Materials and methods
Samples and sources
Subjects were recruited from the most commonly screened populations for HIV antibodies in the real world. According to the Centers for Disease Control and Prevention (CDC) HIV Sentinel Surveillance Implementation Plan, the subjects of this study were categorised into four groups based on HIV-related risk behaviours as follows: (1) The key population included female sex workers (FSWs) and persons with injection drug use (IDU)—FSWSs defined as women currently involved in the commercial sex trade and IDU defined as a person who injects opioids (mainly heroin) and has had a positive urine test for morphine in the last month. FSWs and persons with IDU were sampled and surveyed at the place of sex trade and methadone clinics, respectively. (2) The vulnerable population, in this study, were pregnant women (PW), defined as women undergoing maternal healthcare in preparation for childbirth, who were sampled and surveyed at maternity units in general hospitals or women’s and children’s hospitals. (3) In this study, the general population were students enrolled in tertiary institutions (STUs) who were sampled and surveyed at the school dispensary. (4) The subjects undergoing voluntary HIV counselling and testing (VCT) were sampled and surveyed at the CDC’s HIV testing clinic.
PW are routinely screened for HIV, and women receiving care during pregnancy were recruited from women’s and children’s hospitals. Subjects undergoing VCT were consulted or referred to provincial CDC VCT clinics. This study was conducted from 1 August 2020 to 31 September 2020. No researcher knows whether the subjects were infected with HIV before testing because of previously reported cases that were excluded through the ID card system.
To improve the external validity and to match the characteristics of the real-world HIV screening population, no strict inclusion or exclusion criteria were set for this study; only the following requirements need to be met concurrently: (1) the subject should be at least 18 years of age and in full civil capacity; (2) the subject should have signed the informed consent form and volunteered to participate in the study as a subject; (3) the subject should provide truthful identifying information, such as a driver’s license or identification card, to ensure the subject and the sample are unique and to exclude previously reported HIV cases. Researchers informed subjects of the purpose, methods, potential harms and personal privacy issues of this study in detail before informed consent forms were signed. Following the signing of the informed consent form, each subject was required to be taken three samples, a whole blood sample, a fingertip peripheral blood sample and a urine sample, and to complete the questionnaire after sampling.
The urine rapid test reagent area under the curve (AUC) was predicted to be between 0.85 and 0.98, and the confidence level (1-alpha), CI width, sample dropout rate and screening sample size were set to 0.95, 0.10, 5% and 2500 cases, respectively, requiring a positive sample size of 5–34 cases as estimated by the PASS 2015 software package.
Urine and blood sample testing methods
Three HIV antibody test reagents were used in the study: (1) Reagent A, named the Urine HIV-1 Antibody Rapid Test Kit (colloidal gold), was packaged as a rapid test kit and manufactured by Wantai (20193400550); (2) Reagent B, named Determine HIV1/2 (colloidal selenium), was packaged as a rapid test kit and manufactured by Abbott (20163400427); and (3) Reagent C, named GENscreen ULTRA HIV Ag-Ab (ELISA), which was manufactured by Bio-Rad (72 388C).
HIV antibody tests were divided into on-site tests (for Reagents A and B) and laboratory tests (for Reagent C only). Reagents A and B were used to test for HIV-1 antibodies in urine samples and peripheral blood samples taken from fingertips, respectively. Reagent B is the most common testing method for HIV-1 antibodies in VCT clinics. Urine and venous blood samples were collected from the study subjects using a 100 mL urine cup and a 4 mL EDTA vacuum blood collection tube for Reagents A and C, respectively.
Reagent A and B results were simultaneously identified and recorded by two trained practitioners, and the results were classified as negative, positive or invalid within a specified time frame, according to the reagent instructions. If the two practitioners disagreed on the identification of the same reagent, they uploaded an electronic photo of the reagent, and the result was judged by the quality control team. The anticoagulated blood samples were transferred to the local CDC HIV confirmation laboratory and tested for HIV-1 antibodies under controlled conditions by Reagent C immediately, which was used as the reference method in the study.
All reagents were used in strict accordance with the manufacturer’s instructions, and if any samples from the same participant were positive, the whole blood sample was tested again in the HIV confirmation laboratory and confirmed by both ELISA and western blotting (WB), according to the diagnostic criteria of the Chinese Guidelines for Diagnosis and Treatment of Human Immunodeficiency Virus Infection/Acquired Immunodeficiency Syndrome (2020 edition). Three laboratories with HIV confirmation qualifications participated in the study, including the HIV confirmation laboratories of Guangxi Provincial CDC, Guigang CDC and Liuzhou CDC.
Data management and statistical analysis
The subjects’ information, including basic information such as their name, sex, date of birth, occupation type, education level and ethnicity, as well as their willingness regarding HIV-1 antibody testing methods, purchase channels, acceptable prices and self-tests, were collected through questionnaires.
The main data management and statistical software used in this study included EPIDATA V.3.1, Microsoft Excel 2019, R V.4.1.0, R Studio V.1.4.1103 and IBM SPSS V.26.0. The sensitivity, specificity, receiver operator characteristic (ROC) curve and AUC were used to assess the diagnostic validity of the urine HIV-1 antibody reagents in the on-site screening of different populations; these processes are synchronised in the ROC analysis module of SPSS and the pROC package of the R language. The two-step cluster analysis method in SPSS was used to evaluate the intentionality and user profiles of the study subjects regarding HIV antibody reagent types, acceptable prices, purchase channels and self-tests. The level of statistical significance was set at α=0.05.
The information recorded in the paper questionnaire were entered in pairs using EPI DATE V3.1 and compared for consistency, with key information (ID information, age, sex, population category, education level, willingness to use reagents, etc), HIV antibody test results and other auxiliary information, with consistency levels of 100%, 100% and 99.5%, respectively.
Patient and public involvement
This study was mainly completed by Guangxi CDC, with Guigang CDC, Luzhai CDC and Binyang CDC as the specific implementors of the study. The public and patients (mainly potential patients in this study) were not directly involved in the design and implementation of this study. However, the findings of this study may have some influence on local HIV-related public health strategies in Guangxi, such as promoting non-invasive urine testing reagents for HIV screening in the general population to increase its acceptability and adopting more sensitive and specific methods for screening high-risk populations to find HIV-infected individuals at the early stage.
Results
Basic information about the subjects
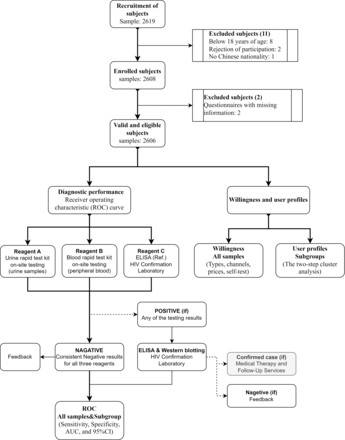
A total of 2606 valid and eligible samples were collected from the FSWs, persons with IDU, PW, STUs and subjects undergoing VCT included in this study, with 202 (7.7%), 304 (11.7%), 1000 (38.4%), 1000 (38.4%) and 100 (3.8%) collected samples, respectively. No adverse events were reported. The flowchart is presented in figure 1. The basic information of each population subgroup are shown in table 1.
The flowchart of this study. The flowchart illustrated the line and methodology of this study. AUC, area under the curve.
The basic information of the 2606 FSWs, persons with IDU, PW, STUs and subjects undergoing VCT in the sample
Consistency of the results of the three reagents
Reagents A and B both showed quality control bands in the 2606 samples tested, and no reagent invalidation occurred. The results of the three reagents are shown in table 2.
The performance of two HIV-1 antibody reagents in field testing (n (%))
The number of probable HIV-positive individuals detected by Reagents A, B and C was 49, 51 and 51, respectively. Of these, 51 individuals with HIV-positive samples detected by Reagents B and C were confirmed to show HIV positivity by both ELISA and WB tests. Of the 49 HIV-positive samples detected by Reagent A, 47 were eventually confirmed to show HIV positivity. Of the three PW diagnosed with HIV by Reagent A, two were misdiagnosed.
The results of Reagent A were fully consistent with those of the reference method for the FSWs (kappa=1.000, p<0.001) and persons with IDU (kappa=1.000, p<0.001), with kappa values of 0.499 (p<0.001) and 0.908 (p<0.001) in PW and subjects undergoing VCT, respectively. The results of Reagent B were fully consistent with those of the reference method, and there were no missed or misdiagnosed cases, as shown in table 3 and online supplemental table 1.
Supplemental material
Supplemental material
Supplemental material
Consistency check of two HIV-1 antibody reagents in diverse subgroups*
Diagnostic performance
The overall sensitivity of Reagent A was 92.16%, the specificity was 99.92% and the AUC was 0.960 (95% CI: 0.952 to 0.968, p<0.001) for the 2606 on-site tests. Reagent B showed identical results to the reference method in the 2606 on-site assays (AUC, 1.000; 95% CI, 0.999 to 1.000, p<0.001), and the overall performance of Reagent A was slightly lower than that of Reagent B (z=2.083, p<0.05), as presented in table 4 and online supplemental table 2. The ROC curves of the two reagents are shown in figure 2.
The receiver operator characteristic curves for Reagents A and B in the 2606 subjects*
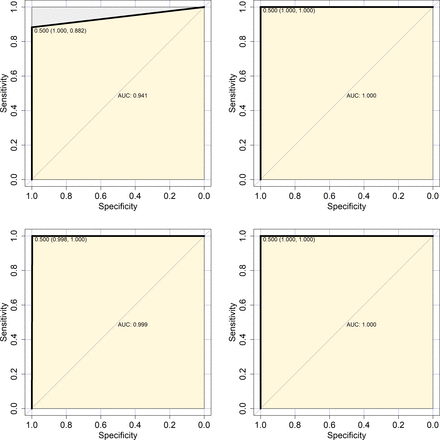
The receiver operator characteristic curves of reagents A and B in 2606 samples. ROC curves for urine rapid test reagents and blood rapid test reagents. AUC, area under the curve.
Reagent A showed good performance in the on-site application for persons with IDU (AUC, 1.000; 95% CI, 1.000 to 1.000, p<0.001), FSWs (AUC, 1.000; 95% CI, 1.000 to 1.000, p<0.001) and PW (AUC, 0.999; 95% CI, 0.997 to 1.000, p<0.001), but the performance differences in each application setting were significant (z=2.908, p<0.005), as shown in (table 5) and online supplemental table 3online supplemental table 3. The ROC curves of the different application settings are shown in figure 3. In this study, the false-negative rate of Reagent A in the subjects undergoing VCT was 6.25% (2/32), and the false-positive rate (FPR) in PW was 0.20% (2/999).
The receiver operator characteristic curves for Reagent A in each group*
The ROCs of urine HIV-1 antibody reagent in VCTs (A), IDUs (B), PW (C) and FSWs (D) groups. ROC curves of urine rapid test reagents in different population subgroups. ROCs, receiver operator characteristics; VCTs, voluntary HIV counselling and testing; IDU, injection drug use; PW, pregnant women; FSWs, female sex workers; AUC, area under the curve.
The AUC of Reagent A in the on-site application for subjects undergoing VCT was 0.941 (95% CI: 0.876 to 0.978, p<0.001). We further dissected and reviewed the causes of this problem: Of the four subjects undergoing VCT with inconsistent results between Reagent A and the reference method, two were men who have sex with men (MSM) who are regularly tested at non-governmental organisations (NGOs) and were recently determined to have HIV-1 antibody positivity, which we speculate may have been due to recent infection. The other two subjects were HIV-infected individuals receiving HAART who requested recertification reports from the subjects undergoing VCT for referral to hospitals in other provinces for treatment.
Willingness regarding and cluster analysis of HIV-1 antibody reagents, prices and channels among different populations
The willingness regarding HIV-1 antibody test reagent types (χ2=430.498, p<0.001), purchase channels (χ2=494.970, p<0.001), acceptable prices (χ2=152.710, p<0.001) and self-tests (χ2=245.966, p<0.001) were significant among the different subgroups, as presented in table 6.
Acceptance of HIV-1 antibody testing methods, access and prices in different populations
The two-step cluster analysis models showed that the ‘acceptable price’ had the greatest influence on STUs (Pi=1.000) and PW (Pi=1.000), the ‘purchase channel’ had the greatest influence on subjects undergoing VCT (Pi=1.000) and persons with IDU (Pi=1.000) and the ‘reagent types’ had the greatest influence on FSWs (Pi=1.000), as presented in table 7.
The user profiles of different populations regarding HIV-1 antibody testing methods, channels and prices
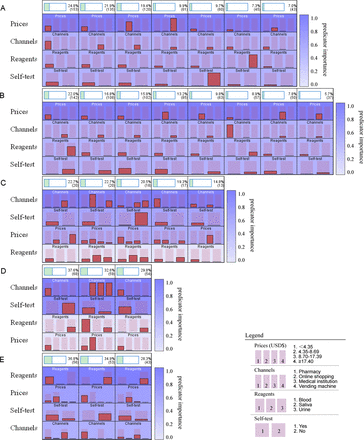
The user profiles of STUs, PW, subjects undergoing VCT, persons with IDU and FSWs were classified into 7, 8, 5, 3 and 3 patterns, respectively. The main patterns of the five populations were as follows and are presented in figure 4: ‘priced <US$4.35, purchased at a pharmacy, blood reagents and willing to self-test’ for STUs; ‘priced <US$4.35, purchased at a medical institution, urine reagents and non-self-testing’ for PW; ‘purchased at a medical institution, willing to self-test, priced between US$4.35 and $8.69 or >$17.40 and blood reagents’ for subjects undergoing VCT; ‘purchased at a medical institution, willing to self-test and blood reagents’ for persons with IDU; and ‘blood reagents, priced at US$4.35–US$8.69, willing to self-test and purchased at medical facilities’ for FSWs.
The user profile patterns of subjects in the two-step cluster analyses, the patterns of STUs (A), PW (B), subjects undergoing VCT (C), persons with IDU (D) and FSWs (E). The user profiles of different population subgroups by two-step cluster analyses.
Discussion
Due to obvious advantages such as non-invasiveness and convenience,15 urine testing for HIV antibodies began in the 1990s, and their diagnostic performance has been confirmed in many studies.16–18 Urine HIV antibody tests have been used in practice for more than a decade,19 and their convenience has been further promoted in recent years with the advent of colloidal gold rapid test kits.12 20 These rapid test kits further enhance the convenience of HIV antibody testing by eliminating the requirement for centralised testing in specialised infectious disease laboratories. However, few studies have reported on the diagnostic performance of rapid urine HIV antibody test kits for practical application in large, complex populations in the real world.
The NSTMP is considered to be the most important scientific and research project in China. Its infectious disease prevention and control projects have been carried out in Guangxi for decades to assess the key issues in the HIV epidemic,21 22 including the low willingness of the population to be screened and the high mortality rate in rural areas due to late HIV detection and diagnosis.23 24 We conducted the study to estimate the diagnostic validity and acceptance of a rapid urine HIV antibody test kit in different populations. As far as we know, such studies are rarely reported.
In this study, based on real-world samples, we found that urine HIV antibody rapid test kits showed satisfactory sensitivity, specificity and ROC curves, especially in high-risk populations such as persons with IDU and FSWs. Commercial heterosexual infections are the main transmission route of HIV in Guangxi, and as a high-risk population, FSWs are a key node in this transmission route.25 26 Both persons with IDU and FSWs are high-risk groups for HIV, and currently, sentinel surveillance and special investigations are the primary public health strategies for identifying HIV-positive patients in high-risk populations. ELISA is the major approach to test for HIV antibodies, which requires the collection of venous whole blood samples from study subjects and transportation to a dedicated HIV laboratory at the CDC for cryopreservation and testing.
In contrast, urine testing offers greater advantages in terms of convenience and timeliness. The administration of injection drugs requires regular urine sample collection for recent opioid, methamphetamine and ketamine abuse, and efficiency and subject acceptance can be improved if urine HIV antibody testing is also conducted instead of blood testing. However, the sentinel surveillance and special investigation of some high-risk groups for HIV infection also require testing for HCV and syphilis,27 28 and the single function of the current urine HIV rapid reagent test limits its applicability.
In practice, physicians treating subjects undergoing VCT are dealing with a very complex population, which is even more complex than the high-risk population. In this study, we routinely tested subjects for blood HIV antibodies and additionally used urine reagent strips to evaluate their performance under complex practice conditions. The urine rapid test kit showed four false-negative cases among 100 subjects undergoing VCT; two were MSM with new infections detected by regular testing at NGOs, and two were patients receiving in-treatment HAART. In the present study, the ROC curve of the urine rapid test kit could have been affected by these false-negative cases if the routine VCT consultation procedure had been followed, and similar false-negative results had been found in some previous studies.14 29 It should be added that the urine reagent’s instructions stated that samples from HIV-infected individuals in the window period or those receiving treatment may yield false-negative results.
Considering the complexities and psychologically protective behaviours of some subjects undergoing VCT, it may be more appropriate to choose an antigen-antibody combined reagent with higher sensitivity and specificity to reduce the possibility of false negatives in some cases where it is difficult for physicians treating these subjects to obtain true and accurate information.30 31 Some subjects with significant psychological fear of HIV but no high-risk exposure may consider using non-invasive urine reagent strips to reduce trauma and receive psychological counselling.
Despite some limitations, urine rapid test kits can be offered as an option for HIV self-testing in high-risk populations such as MSM, FSWs and persons with IDU who require regular testing due to their operability, non-invasiveness and safety; these test kits can have a positive effect on increasing subjects’ willingness to accept and participate in screening.13 32
Previous studies have evaluated urine HIV antibody reagents for general population screening, but this approach required centralised testing by qualified laboratories.20 33 Combined with the internet platform and logistics industry, rapid test kits with urine reagent strips can improve operability through anonymous testing, which may be able to further expand the coverage of general population screening.
In areas with high HIV prevalence, maternal HIV screening helps to identify HIV-infected PW at an early stage and provides timely drug interventions to interrupt mother-to-child transmission,34 which has a positive effect on reducing vertical transmission.35 36 Urine reagent strips showed satisfactory ROC curves in maternal HIV-1 antibody screening, but there were two false-positive tests out of 1000 tests. The reasons for occasional false-positive HIV antibody tests in PW need to be further investigated, and similar occasional occurrences have previously been reported in ELISA screening tests.37 Overall, the FPR of urine rapid test reagents in the PW population is acceptable given the considerable advantages of the non-invasive operation. No positive case was found in the STUs, which we believe is related to the very low prevalence of HIV infection in this population. Thus, the validity of the urine rapid reagent in STUs requires a larger sample size in future studies.
User profiles are the behavioural characteristics of a customer group in selecting or using a product, which is one of the hot analytical approaches in e-business. The current study innovatively applied user profiles to assess the characteristics and tendencies of different population subgroups when choosing reagents for HIV testing. We found that STUs and PW preferred reagent prices below US$4.35, which may be related to the lack of financial income for STUs and the higher cost of childbirth, resulting in price sensitivity for these two groups. We also observed a higher willingness to self-test among the student population, which may be related to the extensive HIV propaganda work carried out in colleges and universities in the past decade.38 39
The low willingness to self-test among persons with IDU and FSWs may be related to the fact that local CDCs conduct free HIV, HCV and syphilis testing for such high-risk populations several times per year. At the same time, persons with IDU and FSWs enrolled in long-term health interventions develop trusting relationships with the CDC, so they are more inclined to choose the medical institution channel and blood reagents. In this study, FSWs preferred urine HIV reagents, which may be related to the non-invasive operation of the rapid test kits. Although the diagnostic performance has been proven in some studies,40 a low percentage of subjects in this study chose the oral secretion HIV antibody test kit, probably due to its expensive price and complicated operation.
People undergoing VCT were more likely to have their HIV antibodies tested in medical institutions, had the highest willingness to undergo self-testing and were also willing to accept more expensive reagents. However, for subjects undergoing VCT, we speculated that their acceptance of HIV-1 antibody testing options, particularly regarding price, may be influenced by factors such as the reason for seeking medical services and psychological status, as all HIV antibody tests conducted in the VCT centres were free of charge.
There were limitations in this study. First, no positive samples were identified in the STUs, and therefore, ROC curves could not be drawn for this subgroup. Second, patients receiving HAART treatment and MSM in the window period were included in the VCT subgroups, which is not consistent with the recommended suggestions for the use of urine HIV reagents; however, this is a complexity that doctors treating subjects undergoing VCT face every day. Despite these limitations, this study evaluated the diagnostic validity of HIV urine rapid test kits in a complex real-world setting and provided some valuable data for the practical application of urine reagent strips.
Conclusions
Overall, the rapid urine test kits showed good diagnostic validity in practical applications, despite a few cases involving misdiagnosis and underdiagnosis. We recommend that physicians providing testing services to subjects undergoing VCTs should carefully select HIV testing reagents based on each subject’s situation.
Data availability statement
Data are available upon reasonable request. The original database for this study contains private information about the study participants. For non-commercial use and reasonable purposes, anonymised data of the current work can be obtained from the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the Ethics Committee of the Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention (approval number GXIRB2019-0047). Participants gave informed consent to participate in the study before taking part.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
HL, HC and SL contributed equally.
Contributors HL, HC, SL, YR, QZ, GL and ML contributed to the conception and design of the study. HL, GT, WC and SZ organised the database. HL and YR performed the statistical analysis. HL, HC and SL wrote the first draft of the manuscript. XP, JL and XG wrote sections of the manuscript. HL, HC and SL contributed equally to the current work. All authors contributed to the manuscript revision and read and approved the submitted version. GL is responsible for the overall content as guarantor.
Funding This work was supported by the National Natural Science Foundation of China (82160636 and 82260670), Guangxi Natural Science Foundation Project (2020GXNSFAA159020), Guangxi Key Laboratory of AIDS Prevention Control and Translation (ZZH2020010), Guangxi Key Research and Development Project (AB19245044), Guangxi Bagui Honor Scholarship, Ministry of Science and Technology of China (2022YFC2305200 and 2018ZX10715008) and Guangxi Medical and Health Key Discipline Construction Project.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.