Article Text
Abstract
Introduction Cardiac rehabilitation (CR) can reduce cardiovascular mortality and improve health-related quality of life. In the United Kingdom, patient uptake of CR remains low (52%), falling well short of the target in the 2019 National Health Service long-term plan (85%). Mobile health (mHealth) technologies, offering biometric data to patients and healthcare professionals, may bridge the gap between supervised exercise and physical activity advice, enabling patients to engage in regular long-term physically active lifestyles. This randomised controlled trial (RCT) will evaluate the feasibility of mHealth technology when incorporated into a structured home-based walking intervention, in people with recent myocardial infarction.
Methods and analysis This is a feasibility, assessor blinded, parallel group RCT. Participants will be allocated to either CR standard care (control group) or CR standard care+mHealth supported exercise counselling (mHealth intervention group). Feasibility outcomes will include the number of patients approached, screened and eligible; the percentage of patients who decline CR (including reasons for declining), agree to CR and consent to being part of the study; the percentage of patients who enrol in standard CR and reasons for drop out; and the percentage of participants who complete clinical, physical and psychosocial outcomes to identify a suitable primary outcome for a future definitive trial.
Ethics and dissemination The trial was approved in the UK by the Northwest—Greater Manchester East Research Ethics Committee (22/NW/0301) and is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Results will be published in peer-reviewed journals and presented at national and international scientific meetings.
Trial registration numbers NCT05774587
- Coronary heart disease
- Myocardial infarction
- Clinical physiology
- Health informatics
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The MOTIVATE-CR+ intervention may increase the uptake of cardiac rehabilitation (CR) by bridging the gap between discharge and the start of supervised CR.
The MOTIVATE CR+ intervention potentially allows patients who recently experienced a myocardial infarction (MI) to codesign a personalised and progressive walking programme with the support of an exercise specialist.
The MOTIVATE CR+ intervention potentially enables participants to communicate regularly with an exercise specialist and gain feedback on the exercise they complete.
The MOTIVATECR+ intervention is not embedded within current cardiac rehabilitation landscape, as such, future work will be needed to address how the intervention could fit within service structures.
Introduction
Cardiac rehabilitation (CR) is a clinically and cost-effective intervention, reducing cardiovascular (CV) mortality and unplanned hospital admissions in addition to improving health-related quality of life (HrQoL).1–4 Despite this, in the UK, the National Audit of Cardiac Rehabilitation estimates that only 52% of eligible patients start CR (defined as uptake).5 A key milestone within the National Health Service (NHS) long-term plan is to increase uptake of CR from 52% to 85% by 2028.6 Reducing the time between hospital discharge and the start of CR in general is essential to meet this milestone; the current recommendation is <28 days postdischarge, but the range in clinical services is 3–111 days.5 Failure to begin CR within 28 days reduces uptake, with recent data suggesting 10 753 patients per year do not take up CR due to a delay in service provision, equating to a loss of 3936 years of life expectancy.7 A possible solution to increase uptake of CR may be to bridge the gap between hospital discharge and the start of supervised CR with remote physical activity (PA) counselling supported by mHealth technology that provides biometric feedback and coaching to patients and health professionals.
The emergence of mobile technologies and wearable sensors has enabled real-world monitoring through mobile health biometrics (mHealth).8 Devices incorporating biometrics such as heart rate (HR) could be a potential solution to bridge the gap between general PA advice on discharge and supervised exercise. HR monitors provide objective personalised data that account for age, body mass and fitness9 and are related to exercise intensity regardless of the type of activity being performed.10 Current research studies (https://www.motivateljmu.com/about) in healthy sedentary individuals, people with newly diagnosed type 2 diabetes and stage 4 CR have explored the acceptability and efficacy of exercise and PA counselling programmes supported by mHealth technologies that provide biometric feedback and coaching to patients and health professionals.8 11 Biometric data such as HR are recorded through a wrist worn fitness tracker to inform exercise counselling delivered by healthcare professionals. Recently, we have shown that the use of mHealth supported counselling leads to adherence of 113%±68 (participants exercised more often than prescribed) and is superior to self-directed web-based exercise in sedentary office workers at risk of CV disease.12
Virtual home-based CR has emerged as an alternative to supervised in-person CR usually delivered in a hospital or leisure centre.13 In the UK, self-directed virtual web-based exercise for CR has been developed but is not used as part of CR standard practice and does not currently allow individualised exercise prescription, biometric monitoring or coaching.14–16 Studies have shown that CR can be effectively delivered remotely using mHealth technologies with biometric monitoring.17 18 While these studies were conducted to compare supervised in-person CR and remote CR, they demonstrated that mHealth technology is acceptable in this patient group.17 18 No study to date has examined whether remote CR with biometric monitoring can be employed as an immediate posthospital discharge intervention to increase uptake of CR.
Study aims
The primary aim is to conduct a feasibility study to evaluate a model where mHealth technology supports a remote home-based PA and counselling intervention immediately posthospital discharge to increase uptake to CR.
The specific objectives are:
Obtain patient demographics and screening, eligibility, recruitment and drop-out rates.
Estimate precision of outcome measures: uptake, time between discharge and start of CR, adherence, CV risk profile, HrQoL.
Assess acceptability of the mHealth PA and counselling intervention.
Assess feasibility and acceptability of outcome measurements and conducting randomised controlled trial (RCT).
Determine availability and completeness of economic data.
Methods and analyses
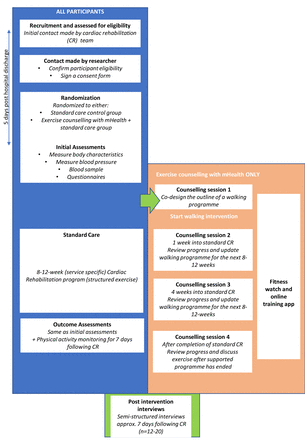
This is a feasibility, assessor blind, parallel group RCT. Participants will be randomised to either CR standard care (active control group) or CR standard care+mHealth supported exercise counselling (mHealth intervention). Outcomes assessment will be completed two times: (1) immediately posthospital discharge, before any intervention and (2) after CR (figure 1). To minimise participant burden and ensure timely completion, outcome measures will be undertaken remotely. The trial protocol adheres to Recommendations for Interventional Trials and the Template for Intervention Description and replication guidelines.19 20
Study flow diagram and participant pathway.
Study setting and recruitment plan
Recruitment (n=60) will take place at three UK CR sites; North-West England (n=20), West Midlands (n=20) and North-East England (n=20) commencing May 2023 for 12 months. The trial will end (last data collection from the last participant) in June 2024. The participant information sheet (PIS) will be added to hospital discharge packs, and patients will then be contacted via telephone by a CR team immediately postdischarge as part of routine care (within 48 hours). During this contact, the CR team will discuss the study. If the patient expresses an interest in the study, the CR team will request verbal consent to pass contact details to the research team, who will contact interested participants via telephone (or video call) to discuss the PIS (provided by CR team), ask any questions and confirm eligibility criteria. Participants will be consented and screened, which involves a medical history, details of current medications, current PA and exercise behaviour (figure 2).
Schematic of the experimental design. CR, cardiac rehabilitation, CS, counselling session; n, number.
Eligibility criteria
Eligible participants will have been referred to CR with a recent clinical diagnosis of MI and have been discharged within the last 5 days.
Detailed inclusion criteria
Participant is willing and able to give informed consent for participation in the study.
Men or women.
Over 18 years old.
Post-MI.
Postpercutaneous coronary intervention patients.
Referred for CR.
Detailed exclusion criteria
Acute or unstable health conditions.
Coronary artery bypass graft surgery.
Unable to participate in self-management programmes because of medical care needs.
Absolute contraindications to exercise.
Unable to operate or own mobile/smartphone devices/lack of internet access.
Declined CR standard care.
Allergies to the watch materials.
Atrial fibrillation or other arrhythmia preventing accurate HR.
Randomisation and blinding
Randomisation will be computer generated and the code held by an independent person blinded to the groups to maintain allocation concealment according to the Standard Protocol Items: Recommendations for Interventional Trials guidelines.19 Due to the nature of the intervention, blinding the participants is not possible. Following the initial screening process, participants will be randomly allocated to the two study groups (active control or mHealth intervention) and informed by telephone/email (patient preference).
Outcome measures
Primary outcome
Outcome measures (table 1) will be taken using remote ‘home-based’ solutions, which do not require travel or in-person contact. Throughout the study, information will be collected on (1) the total number of patients screened, eligible, approached, (2) the % of patients who (a) decline CR (including reasons for declining); (b) agree to CR and (c) consent to being part of the study; (3) the % of patients who take up standard CR following the mHealth intervention and reasons for drop out of CR before the end of the intervention (if provided) and (4) the % of participants who complete outcome assessments and reasons for drop out (if provided).
Primary objectives and outcome measures
Secondary outcomes
Age, sex, ethnicity, reason for enrolment into CR, centre referred to, education and employment status will also be collected via an initial screening telephone call. Immediately following consent and randomisation, participants will be mailed (direct to patients preferred address) all necessary assessment resources, including written and video guidance on how to complete the assessments (https://www.motivateljmu.com/cr.) within the 1–5-day timeframe. Participants will then receive a phone/video call from the research team to (1) discuss the testing protocol and allow patients to ask any questions they may have and (2) gain patients current medication information (current medications and dose) and ethnicity. On the day of testing, a member of the research team will be available via phone/video call to provide support where required. Using this approach, we expect participants to begin the mHealth intervention within a maximum of 5 days postdischarge.
Exercise adherence and habitual PA
Adherence to home-based exercise prescription is difficult to measure using one method. Accordingly, the feasibility of three outcome measures will be assessed:
Device-derived assessment of exercise sessions: the number of planned structured exercise sessions completed along with the duration and intensity of each session will be assessed via optical HR monitoring (photoplethysmography). The mHealth group will use the Polar Ignite 2 fitness watch and the Polar Verity sense optical HR monitor (Polar Electro, Finland) provided as part of the intervention. The active control group will be provided with a Polar Verity Sense optical HR monitor for the duration of the trial, to wear during planned structured exercise sessions (eg, structured CR session). The Polar Verity Sense records HR but gives no real-time/historical feedback to participants. As such active control participants will be blinded to the HR throughout.
Device-derived PA: key metrics of PA will be assessed using a wrist-worn triaxial accelerometer (GENEActiv, Activinsights, Kimbolton, Cambridge, UK) during the final 7 days of the intervention period immediately after follow-up testing. Before sending to the participant, the accelerometer will be initialised and set by the research team to start and finish recording at specific dates. Data will be downloaded using manufacturers’ software and processed in R (R Core Team, Vienna, Austria) using the open-source GGIR software package (http://cran.r-project.org).
Survey-reported exercise behaviour will be evaluated using the Godin Leisure Time Exercise Questionnaire (GLTEQ) at baseline and postintervention. The questionnaire will be administered using an online platform (Qualtrics, Provo, UT) survey.
Baseline and post intervention testing
Testing will take place in the morning between 6:00 and 10:00 and should take approximately 45 min. Participants will be fasted overnight and instructed to abstain from caffeine, alcohol and moderate/vigorous exercise the day before testing. Participants will be asked to drink a glass of water immediately before the measures are taken.
Anthropometrics
Participants will be sent a measuring tape (Seca 201, Germany) to be used for assessing height and waist circumference. Waist circumference will be measured in triplicate at the level of the umbilicus. Previous work suggests a strong correlation between self-measured and technician-measured height and weight (Salter, UK)21 and waist circumference, measured at the umbilicus.22
Blood pressure
Patients will be asked to rest in a seated position for 10 min before measuring their blood pressure using an automated blood pressure monitor validated by the British and Irish Hypertension Society (UK, Salter BPA-9200-GB; Canada, Bios BD215). Patients will wrap the blood pressure cuff around their non-dominant arm. Blood pressure will then be measured in triplicate, leaving 1 min between successive measurements. Self-measured blood pressure is a validated approach for monitoring blood pressure, endorsed by the American Heart Association and American Medical Association.23
Blood sampling
Patients will then collect a 500 μL blood sample from a finger prick, using a self-administered commercial blood collection kit, via Royal Devon and Exeter NHS Foundation Trust (MonitorMyHealth.org.uk) in accordance with predefined procedures. Patients will be asked to post the envelope on the same day as collection. Due to the time-sensitive nature of the sample, patients will be sent a text/email (patient preference) to remind them to post the sample. Blood samples will be posted by participants to the Royal Devon and Exeter NHS Foundation Trust. Samples will be analysed for HbA1c, total cholesterol, high density lipoprotein (HDL)/ low density lipoprotein (LDL) cholesterol and triglycerides by the Clinical Chemistry department at the Royal Devon and Exeter Hospital. Donor information will not be available to the team at Royal Devon and Exeter Hospital as samples will be sent using pseudonymised sample codes only; however, members of the research team will be able to identify donors via participant numbers. Internal pilot data from the Exeter Clinical Laboratory demonstrate that capillary blood sampling reveals good agreement with standard venous sampling.
Patient questionnaires
All patients will complete online versions of (1) the 5-level EuroQol-5 Dimensions, (2) a study-specific questionnaire assessing healthcare use over the previous 12 weeks, (3) the GLTEQ, (4) the heart disease HrQoL (MacNew) questionnaire and (5) the Behavioural Regulation in Exercise Questionnaire V.2. The questionnaires will be completed using Qualtrics by assessor blind to group allocation. Patients will be encouraged to complete the questionnaires immediately after testing. Should questionnaires not be completed reminders will be sent (text/email dependent on patient preference) following 1 and 3 days. If need be, we will offer to do the questionnaires over the phone or secure video conferencing with patients. No CV assessments will take place due to the remote nature of the testing and the lack of monitoring available for participants during any submaximal physical exertion.
Semi-structured interviews
Process evaluation
A detailed process evaluation will examine the acceptability and feasibility of the intervention and evaluation methods. Semistructured interviews will be conducted postintervention (table 2):
Postintervention feedback on the intervention.
Postintervention feedback on acceptability of the research process.
Details of semi-structured interviews
All interviews will be conducted by another individual on the research team via telephone or video call according to participant preference and will be structured using a topic guide.
Qualitative analysis
Interviews will be audio/video recorded and transcribed verbatim, deductively coded and analysed using the theoretical domains framework enabling challenges and facilitators within the intervention to be identified.24 Transcriptions will be thematically analysed25 and coded either manually or using NVivo V.12TM software. Data will be thematically analysed using reflexive thematic analysis recommendations such as data familiarisation, generating initial themes, coding and finalising patterns of shared meanings underpinned by a central concept, and writing up using data extracts interspersed with researcher interpretations.25 Although the data themes will be created deductively, the patterns of shared meaning will be inductively generated from the data themselves allowing interpretation and researcher contextual awareness to be discussed.25 Member checking will be the final step in analysis, ensuring that interviewed participants have the opportunity to confirm researcher interpretation and add comments that will be incorporated into the final analysis.26 Our aim is to develop a comprehensive understanding of the intervention acceptability, implementation and mechanisms of impact.
Economic assessment
At baseline and postintervention, all participants will complete the EuroQol-5 Dimension questionnaire and a study-specific questionnaire assessing healthcare usage in the last 12 weeks (GP services, specialist care, ambulatory clinics in hospital, physiotherapy and medicines). During the interventions, researcher time per participant will also be recorded in both groups.
Interventions
An initial intervention meeting will be scheduled via preferred virtual platform. All subsequent intervention meetings will be held using this platform. Either a Polar Verity sense HR monitor (standard CR care) or Polar Ignite 2 and Polar Verity sense HR monitor (exercise counselling+mHealth+standard CR care) will be posted to the patient following randomisation (contained within parcel for assessment equipment). Patients in the exercise counselling+mHealth+standard CR care group will also be provided with instructions to download the Polar Flow—Sync & Analyze application from the App V.6 30.06.22 Store (IOS devices) or Google Play (Android devices). The app will be initialised in the first exercise counselling session.
Participants will be given written and verbal instruction regarding contraindications to exercise and will be asked to confirm they are not experiencing any of these prior to their exercise session. If participants are experiencing any of these symptoms, then they must not exercise and inform the research team. Participants will be randomised into one of two groups:
CR standard care control group
Participants will follow CR standard care. Participants have contact (eg, telephone/virtual and/or home visit) with the CR team between discharge and beginning CR. They will begin structured exercise at the time provided by the CR service, the structured exercise service consists of 1–2 supervised exercise sessions per week for 8–12 weeks at the clinical or community centre. Exercise sessions are circuit-based, CV and strength exercise of light to moderate intensity (40%–70% HR reserve (HRR)). Participants can wear an unblinded HR monitor during structured exercise provided by the CR service. As part of the study, they will also always wear the blinded verity sense optical HR monitor provided by the research team.
mHealth-supported exercise counselling+CR standard care experimental group
Participants will codesign a personalised and progressive home-based walking programme, with support from the exercise specialist, which starts immediately following hospital discharge and study measures and continues as an adjunct once/if structured exercise CR begins (table 3). To assist with the transition to independent exercise and to promote long-term adherence, participants will receive four virtual exercise counselling sessions. The first, held within 5 days of discharge, will be used to assess current beliefs/concerns, explore the benefits of exercise and agree on a specific, measurable, achievable, relevant and time-bound PA plan. During this initial phase, participants will be prescribed an individualised (initial duration and intensity of sessions and rate of progression) walking plan. Once structured exercise CR has begun, a second session will be held to discuss progress and refine goals with the aim of progressing the programme. At this time, home-based walking sessions will be performed alongside structured exercise CR sessions to increase adherence in daily life. A third meeting will be held 1 month into CR to discuss progress. A final meeting will occur at the end of CR to review progress and strategies to maintain exercise and PA. Each participant’s exercise programme will differ, but the aim will be to increase exercise intensity and duration throughout the programme with the goal of achieving 150 min of moderate-intensity exercise per week, when combined with structured exercise CR sessions. HR zones of 40%–70% will be calculated using the Karvonen HRR formula27 as identified in The British Association for Cardiovascular Prevention and Rehabilitation (BACPR) guidelines.28
Details of counselling intervention
Behaviour change intervention
Our mHealth technology supported PA and counselling intervention, MOTIVATE, is designed based on the principles of the Capability Opportunity Motivation and Behaviour (COM-B) model of behaviour change.29 An analysis of the intervention components showed that MOTIVATE addressed capability by suitably screening participants and identifying barriers and motivators through goal setting during exercise counselling sessions, alongside increasing the participant’s confidence when completing remotely monitored exercise sessions. Opportunity has been identified through the use of remote feedback and monitoring via text messages. Motivation was addressed using motivational interviewing technique processes, in addition to the removal of the barrier of travelling to an exercise facility, and in combination with using goal setting and feedback text messages.
The experimental intervention will be supported by three mHealth elements: (1) online coaching platform for exercise specialist: (polar flow for Coach, https://flow.polar.com/coach), within the platform, the exercise specialist will build the codesigned exercise programme, specifying the agreed number of sessions per week and prescribing the duration and intensity of each phase, that is, warm up, workout and cool-down. Structured exercise CR sessions will also be recorded, so these can be tracked. Throughout the intervention, the online platform will also provide the research fellow access to the participant data, including daily PA, HR during exercise, rate of perceived exertion (RPE) (CR-10 scale30) and written comments on exercise sessions.
Smartphone app: (Polar Flow—Sync & Analyze) participants will access their walking programme, use the app to track their exercise and PA achievements. All data recorded by the fitness tracker (see details below) will be available within the app, and participants will use the app to provide feedback on each exercise session, including a session RPE (CR-10 scale) and a written comment.
Fitness watch (Polar Ignite 2, Polar Electro): the Polar Ignite 2 fitness watch features a triaxial accelerometer and optical HR monitor. Participants will access preset exercise sessions, designed by the exercise specialist in line with BACPR guidelines and Frequency, Intensity, Time and Type principles, on the device. The prescribed duration and intensity, via HR zones, will be displayed in real time on the watch throughout the exercise session. The watch will also provide live visual and haptic (vibration) alerts, coaching participants to execute the session as prescribed. Progress towards a personalised daily PA target will also be displayed throughout the day on the watch screen. All data recorded on the watch will be synchronised with both the smartphone app and the online platform. For the remainder of the walking intervention (including during CR), messages will be sent weekly. Participants will be able to respond to these comments and programmes will be updated if necessary.
Ongoing communication
Data from the mHealth elements, including participant comments, will be used to facilitate ongoing personalised feedback. During the first month, participants will be asked to provide an RPE and written comments following all exercise sessions, using the smartphone app. The comment will relate to the appropriateness of the session duration and intensity and their enjoyment of the walking programme. After each recorded exercise session, the exercise specialist will then use the participant feedback to send a personalised text message in response to the session. Based on this feedback, the exercise specialist will update the walking programme as appropriate via the online platform. The aim of this initial 1-month period is to refine the exercise sessions to ensure participants have a programme that meets their current fitness and lifestyle requirements. Following exercise counselling, three participants will receive weekly text messages from their exercise specialist until the end of the intervention period.
Exercise specialist
The role of exercise specialist will be assumed by a postdoctoral research fellow in the UK (a Registration Council for Clinical Physiologists registered Clinical Exercise Physiologist with PhD in exercise science). The same exercise specialist will provide support to both arms of the trial.
Study withdrawal
Each participant has the right to withdraw from the study at any time with no obligation to provide a reason. If provided, reasons for withdrawal will be retained, but personal data will be disposed of. In addition, participants may be withdrawn from the study by the research team at any time if the research team considers it necessary for any reason including:
Ineligibility (either arising during the study or retrospectively having been overlooked at screening).
Significant protocol deviation.
Significant non-compliance with treatment regimen or study requirements.
Withdrawal of consent.
Loss to follow-up.
Withdrawal from the study will not result in exclusion of the participant’s data from analysis, including audio recordings that have already been transcribed as all of this data will be pseudonymised. Withdrawn participants will not be replaced. Participants will be asked reasoning behind withdrawal either via email or phone call. The reason will be recorded in the study file. Participants are free to give no reason.
Serious adverse event reporting and management
Participants will be asked if an adverse event (AE) has occurred during the exercise counselling sessions at the start of CR and end of CR, plus during ongoing text message support. Should an AE be reported, an independent clinician will assess the event and the end outcome using the serious AE (SAE) Report Form. The chief investigator (CI) will then report the event to the sponsor using the SAE letter template with suitable outcomes required before continuation of study. SAEs are defined as any AE at any stage in the research participation of the study which
results in death,
is life threatening,
requires hospitalisation or prolongation of existing hospitalisation,
results in persistent or significant disability or incapacity,
is a congenital anomaly/ birth defect.
Life-threatening refers to an event in which the participant was at risk of death at the time of event; it does not refer to an event, which hypothetically might have caused death if it was more severe.
Expected AEs include
Development of unstable angina.
Cough and colds.
Influenza.
Muscle aches and pains.
Muscle strains.
Indigestion.
Constipation.
COVID-19
Data management
Direct access to data will be granted to the research team, authorised representatives from the sponsor and host institution for monitoring and/or audit of the study to ensure compliance with regulations. Paper data will be stored in a locked cabinet at LJMU, only accessible to the PI and postdoctoral fellow. An electronic file containing the link between participants’ name and study number will be stored in a password-protected file and only be accessible to the PI and postdoctoral fellow. A paper copy of this file will also be stored in a locked cabinet in the principal investigator’s office at LJMU. Audio/video recordings which contain personal data will be recorded on a password-protected device and transferred to password-protected storage and deleted from the recording device. All patients will be given a pseudonymised study code. This code will be used for all stored data, including transcripts of interviews and audio recordings. Published quotes will be pseudonymised using this code. Pseudonymised data will be transferred between investigators using an internet-based data transfer portal. This data will reside in a secure server with access restricted to allocated staff. Only the LJMU study team will have access to personal identifiable information.
Interviews will be transcribed and analysed by the team study team at Liverpool John Moores University. Our intended policy is that the study team should have exclusive use of the data for a period of 12 months or until the data is published. Data will be shared with named collaborators during this time. Following this data will be publicly available through the LJMU Data Repository, published under a permissive reuse license. A CC BY NC license will be applied to openly available data, this creative common license permits others to distribute and build on the work for non-commercial purposes.
Sample size calculation
As this is a feasibility study a formal power calculation is not appropriate. The sample size was based on published good practice recommendations for pilot/feasibility studies with the median value for feasibility studies (36 participants per arm) reported in an audit of pilot and feasibility trials registered in the UK clinical research network.31 32 The proportion of eligible patients who consent to participate will be presented by site and overall, along with the proportions in each intervention group completing each follow-up assessment and the reasons for withdrawal. Descriptive characteristics and outcome data will be summarised overall and by intervention group, as mean (SD) for normally distributed continuous variables, median (IQR) for non-normally distributed continuous variables and number (percentage) for categorical variables.
Trial oversight
The quality of the study will be assured through the series of management groups. The trial will be overseen by a trial steering committee (TSC) and operated on a day-to-day basis by a trial delivery group (TDG). The TSC will comprise of experienced academic experts (research team) and patients but does not require and, therefore, will not have an independent chair. The TSC will meet quarterly to discuss progress. The role of the TSC is to provide overall supervision of the trial. In particular, the TSC will concentrate on the progress of the trial, adherence to the protocol, participant safety and consideration of new information. The TSC must be in agreement with the final protocol and, throughout the trial, will take responsibility for major decisions such as need to change the protocol for any reason, monitoring and supervising the progress of the trial, reviewing relevant information from other sources and informing and advising the TDG on all aspects of the trial.
The TDG will comprise of the same research team and will hold monthly meetings to discuss progress. The responsibilities of the TDG will include:
Report to the TSC.
Maintain the trial master File.
Confirm all approvals are in place before the start of the trial at a site.
Provide study materials.
Data management centre.
Give collaborators regular information about the progress of the study.
Respond to any questions (eg, from collaborators) about the trial.
Ensure data security and quality and observe data protection laws.
Safety reporting.
Ensure trial is conducted in accordance with Good Clinical Practice.
Statistical analysis.
Publication of trial results.
Patient and public involvement
Patient and public involvement work was conducted with patients participating in stage 4 CR at one of the sites. Patients (n=9) were given the programmed mHealth technology to use for 12 weeks. Exercise intensity was successfully prescribed and monitored using HR, including in those on beta-blockers, in this group. Eight of nine patients described the intervention as very/extremely helpful in increasing their exercise levels, citing the improved communication with their CR specialists and feedback given by the watch during exercise as facilitators.
One patient representative will be invited on the TSC. They will advise on study information materials to recruit participants to the study. At the end of the project, our patient representatives will contribute to the reporting of the study through reading and reviewing the ‘lay’ sections of the report. They will also be involved in dissemination of research findings through reviewing literature outlining the results before they are circulated.
Ethics and dissemination
The trial protocol has received favourable opinion from the Greater Manchester East Research Ethics Committee (22/NW/0301) in the UK. On study completion, the CI owns the data. On completion of the study, the data will be analysed, and results will be disseminated via publication in clinical and physiological journals, presented at National and International conferences and in the form of feedback sheets or perhaps local articles. Participants will not be identifiable from the results of the study. Pseudonymised data from this study will be made available for sharing with other investigators, after publication of the study’s key papers. Data will be shared through the LJMU Data Repository (http://opendata.ljmu.ac.uk/). This is a secure institutional data repository, which is searchable on the www, it is managed by Library Services. A DOI will be generated for datasets as they are deposited to the repository. Data will be stored in this repository for a minimum of 10 years or for 10 years from the last date of access.
Ethics statements
Patient consent for publication
References
Footnotes
Twitter @kathskth
Contributors HJ, AC, KH, LT, GMc, GM and MC initiated the study design. HJ, GMc, GM, LT and MC are grant holders. All authors contributed to refinement of the protocol and approved the final manuscript.
Funding This research was funded by Heart Research UK NET21-100010.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer-reviewed.